Table 3.
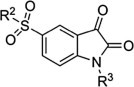
| Compound | R2 | R3 | IC50 (μM) |
|---|---|---|---|
| 8a1 |  |
CH3 | 11.83 ± 1.87 |
| 8a2 | PhCH2 | 67.20 ± 8.50 | |
| 8a3 | β-C10H7CH2 | 82.91 ± 12.91 | |
| 8b2 | PhCH2 | ND | |
| 8b3 | β-C10H7CH2 | ND | |
| 8d2 | 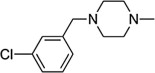 |
CH3 | ND |
| 8d3 | PhCH2 | ND | |
| 8f1 | 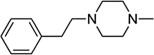 |
β-C10H7CH2 | 13.86 ± |
| 8f2 | CH3 | ND | |
| 8f3 | PhCH2 | ND | |
| 8h1 | β-C10H7CH2 | 5.52 ± 0.33 | |
| 8h2 | CH3 | ND | |
| 8h3 | PhCH2 | ND | |
| 8i2 |  |
β-C10H7CH2 | 14.00 ± 2.472 |
| 8i3 | β-C10H7CH2 | ND | |
| 8j1 |  |
CH3 | 9.91 ± 0.79 |
| 8j2 | PhCH2 | 13.86 ± 2.96 | |
| 8j3 | β-C10H7CH2 | 39.87 ± 0.62 | |
| 8k1 | 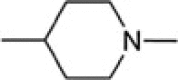 |
PhCH2 | 1.04 ± 0.01 |
| 8k2 | β-C10H7CH2 | 1.69 ± 0.01 | |
| 8k3 | CH3 | 17.82 ± 0.72 | |
| 8m1 | 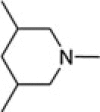 |
PhCH2 | 2.82 ± 0.17 |
| 8m2 | β-C10H7CH2 | 4.70 ± 0.12 | |
| 8m3 | PhCH2 | ND |
ND: not done because of quenching rate >20%.
