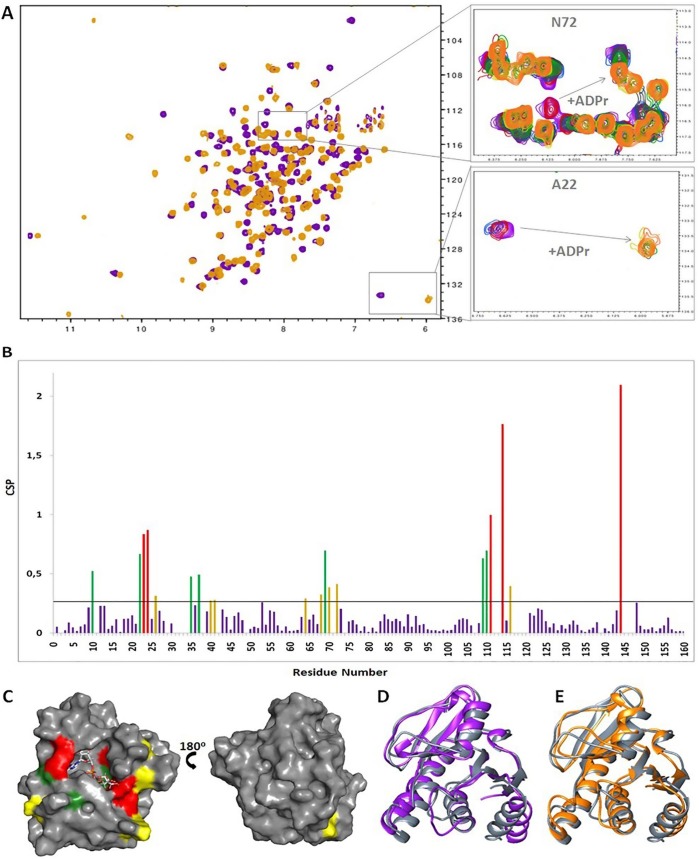
Fig. 2.
Interaction of VEEV macro domain with ADPr. (A) Overlay of 1H–15N HSQC spectra of the VEEV macro domain in the apo (purple) and ADPr bound state (ratio 1:4) (orange). The response of the NMR resonances of A22 (bottom) and N72 (top) to increasing ADPr concentrations. Peaks corresponding to the apo state decrease in intensity as the ones of the bound state increase (purple: free, red: 1:0.250, light blue: 1:0.5, grey: 1:0.750, green: 1:1, yellow: 1:2, orange: 1:4). (B) Chemical shift perturbation (CSP) in response to ADPr binding (ratio 1:4) with threshold value 0.26. Residues with CSP up to 0.2 from the threshold value are colored yellow, residues with CSP from 0.2 to 0.5 above the threshold are colored green and residues with CSP higher than 0.5 from the threshold are colored red. (C) Surface representation of the VEEV macro domain in complex with ADPr. The residues with CSPs above the threshold are mapped onto the surface using the same color code as in B. (D) Superposition of ribbon representations of VEEV macro domain in the apo (purple) and ADPr bound states (grey) as determined by NMR spectroscopy. (E) Superposition of ribbon representations of the VEEV macro domain-ADPr complex as determined by X-ray diffraction (orange) and NMR spectroscopy (grey).