Table 2.
Oxidation of the HPMP analogues 8 with TEMPO/NaClO2/NaClO to give 9, followed by diester removal to yield 7
| Entry | Nucleobase B | Starting compound | Product (yield)a of oxidation | Product (yield)a of the phosphonate deprotection |
|---|---|---|---|---|
| 1 |  |
8a (S)- | 9a (87%) | 7a (68%),b (46%)c |
| 2 | 8b (R)- | 9b (76%) | 7b (50%)b | |
| 3 | 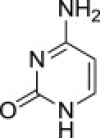 |
8c (S)- | 9c (83%) | 7c (62%),b (39%)c |
| 4 |  |
8d (S)- | 9d (82%) | 7dd (64%),b (44%)c |
| 5 |  |
8e (S)- | 9e (81%) | 7e (42%)c |
| 6 | 8f (R)- | 9f (80%) | 7f (48%)c | |
| 7 |  |
8g (S)- | Complex mixture (no 9g isolated)e | 7g (61%),b (37%)c |
| 8 | 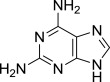 |
8h (S)- | Complex mixture (no 9h isolated) | 7h (38% from 9j)c,f |
| 9 | 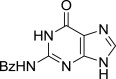 |
8i (S)- | 9i (78%) | 7i (52%)b |
| 10 |  |
8j (S)- | 9j (80%) | N.A.g |
