Table 2.
Cycloaddition of the nitrones 20a-20e and alkenylphosphonates 23–25.
| Nitrone | Nucleobase B | Alkene | Reaction time (h)a | Cis/trans ratio | Yield [%] |
|---|---|---|---|---|---|
| 20a |
23 24 25 |
30 30 30 |
80:20 72:28 89:11 |
27a – 22b; 27a + 28a – 24c 29a – 16b; 29a + 30a – 32c; 30a – 9b 31a – 30b; 31a + 32a – 13c |
|
| 20b |
23 24 25 |
10 16 14 |
74:26 79:21 86:14 |
27b – 3b; 27b + 28b–10c 29b–13b; 29b + 30b–23c 31b–20b; 31b + 32b–26c |
|
| 20c |
23 24 25 |
8 21 8 |
86:14 72:28 70:30 |
27c – 4b; 27c + 28c – 15c 29c – 12b; 29c + 30c – 3.3c 31c – 19b; 31c + 32c – 10c |
|
| 20d |
23 24 25 |
8 10 8 |
71:29 80:20 84:16 |
27d–21b; 27d + 28d–15c 29d – 5b; 29d + 30d–20c 31d–27b; 31d + 32d – 7c; 32d – 2b |
|
| 20e | 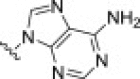 |
23 24 25 |
40 26 21 |
– 80:20 69:31 |
decompositiond –e –e |
Cycloaddition under MW irradiation.
Yield of the pure diastereoisomer.
Yield of the pure mixture of diastereoisomers.
Decomposition of the starting nitrone 20e was observed. The unreacted allylphosphonate 23 was recovered almost quantitatively.
Ratio of diastereoisomeric cycloadducts 29e and 30e as well as 31e and 32e were calculated, however pure isomers could not be isolated from the mixture containing several unidentified products.
