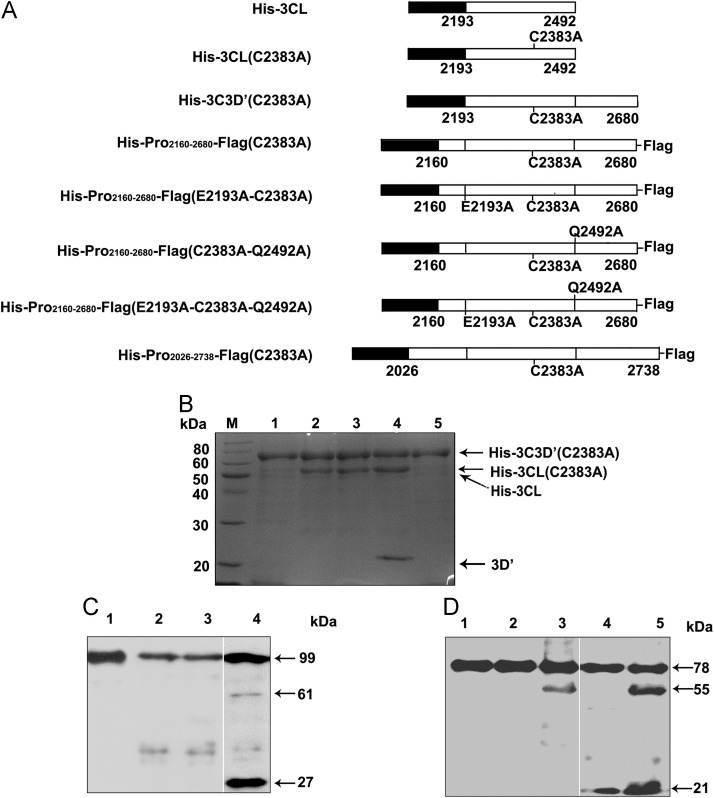
Fig. 6.
Trans-cleavage of the EoV 3CL protease. (A) Schematic illustrations of the wild-type and mutant 3CL proteases and various substrates. (B) The substrate His-3CD′(C2383A) was expressed in E. coli, purified via Ni–NTA chromatography, and then mock-treated for 0 h (Lane 5) or 6 h (Lane 1), incubated with His-3CL (active protease) for 0 h (Lane 2) or 6 h (Lane 4), or incubated with His-3CL(C2383A) (inactive protease) for 6 h (Lane 3). Then, the reaction mix was subjected to 12% SDS-PAGE, and the proteins were stained with Coomassie brilliant blue R250. Lane M, molecular weight marker. (C) The substrate His-Pro2026–2738-Flag(C2383A) was incubated with His-3CL (active) for 0 h (Lane 1) or 6 h (Lane 4), incubated with His-3CL(C2383A) (inactive) for 6 h (Lane 3), or mock-treated for 6 h (Lane 2). The reaction mixtures were separated via 12% SDS-PAGE and then subjected to Western blot analysis with anti-Flag antibody. The arrow indicates the position of the fusion protein or cleavage products. (D) Active His-3CL was used to treat different substrates, including His-Pro2160–2680-Flag(C2383A) for 0 h (Lane 1) and for 6 h (Lane 5) and His-Pro2160–2680-Flag(E2193A-C2383A-Q2492A) (Lane 2), His-Pro2160–2680-Flag (C2383A-Q2492A) (Lane 3), and His-Pro2160–2680-Flag(E2193A-C2383A) (Lane 4) for 6 h. The reaction mixtures were separated via 12% SDS-PAGE and then subjected to Western blot analysis with anti-Flag antibody.