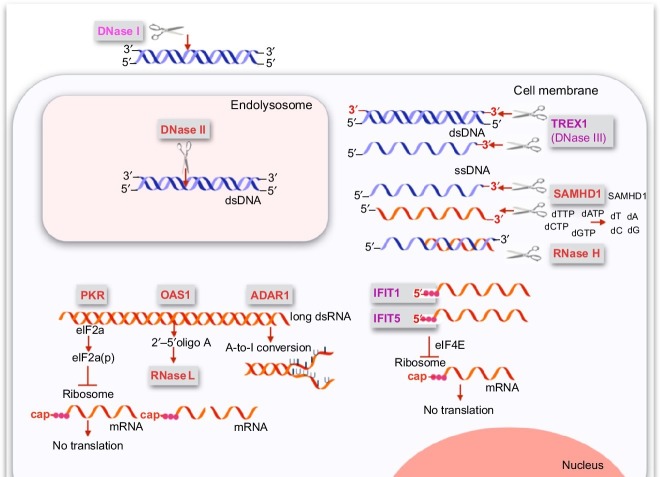
Fig. 5.
Receptors and nucleases restricting function and replication of foreign nucleic acids. This graph provides an overview of the proteins which target foreign nucleic acids without involving the classical immune functions such as the induction of cytokines or the activation of immune cells. Such proteins can directly act on the foreign nucleic acid, or they can elicit pathways indirectly acting on the foreign nucleic acid. The endonuclease DNase I is the most abundant DNase in the extracellular space which degrades DNA down to tetramers. DNase II is the predominant endonuclease in the endolysosomal compartment of cells. The cytoplasmic DNase III (Trex1) is a 3′-to 5′ exonuclease which degrades both double- and single-stranded DNA. The cytoplasmic RNase H recognizes DNA–RNA hybrids and cleaves the RNA in such hybrids. In contrast, RNase L is indirectly activated by oligoadenylates which are formed by OAS1 upon binding to long double-stranded RNA. Furthermore, ADAR1 modifies long double-stranded RNA by A-to-I conversions destabilizing the double strand resulting in changes in the coding sequence of proteins. SAMHD1 depletes the pool of dNTPs which is the prerequisite for DNA formation. SAMHD1 hydrolyzes the triphosphate in dNTPs resulting in deoxynucleosides. At the same time, SAMHD1 has been proposed to be a 3′-exonuclease for single-stranded DNA and RNA. PKR and IFIT1/5 inhibit mRNA translation by phosphorylation of the elF2a and by replacing elF4 in the ribosomal complex, respectively. While PKR is activated by long double-stranded RNA, IFIT1 and IFIT5 bind 5′-triphosphate ends of single-stranded RNA.