Fig. 2.
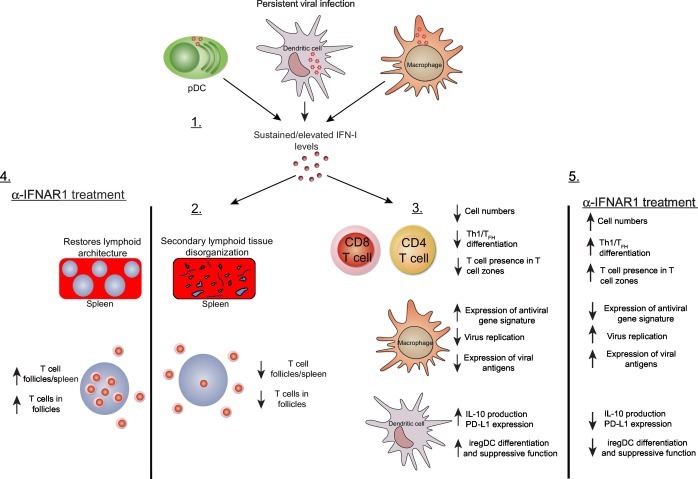
Elevated IFN-I signatures during persistent viral infection support a global immunosuppressive program. 1. Infection with persistent viruses results in elevated IFN-I production and downstream gene signatures, which are maintained throughout the infection even without detectable IFN-I protein levels. 2. At the organ level, elevated IFN-I signatures prevent proper organization of secondary lymphoid architecture, with fewer T cell zones and less recruitment of T cells into T cell zones. 3. Elevated IFN-I signatures promote T cell exhaustion reducing T cells numbers and preventing Th1/TFH differentiation. Upregulation of antiviral gene expression in marginal zone macrophages results in inhibition of virus replication in these cells and suboptimal levels of viral antigen required for triggering antiviral humoral immune responses. IFN-I signaling on dendritic cells promotes sustained expression of the negative immune regulatory molecules IL-10 and PD-L1, promotes the generation of iregDCs, and maintains T cell immune suppression. 4. Blockade of IFN-I signaling during persistent viral infection using an anti-IFNAR1 monoclonal antibody restores lymphoid architecture and promotes T cell migration/residence in T cell zones. 5. Restores T cell function, increases expression of viral antigens in CD169+ macrophages, blunts production of the negative immune regulatory molecules IL-10 and PD-L1, and inhibits differentiation and suppressive function of iregDCs. The above restoration of immune cell functions following IFN-I blockade ultimately results in hastened clearance of the persistent viral infection.
