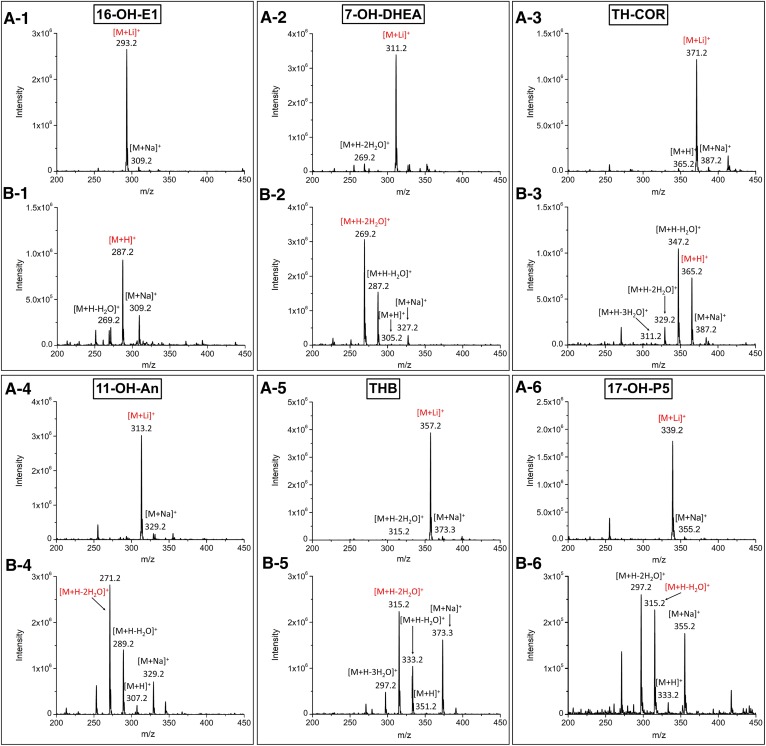
Fig. 2.
ESI-MS spectra of the 3-OH steroids. The mobile phase consisted of solvent A [0.2 mM LiCl in deionized water (A) and 0.1% FA in deionized water (B)] and solvent B (MeOH). 1, 16-OH-E1; 2, 7-OH-DHEA; 3, TH-COR; 4, 11-OH-An; 5, THB; 6, 17-OH-P5. Two hundred nanograms of each steroid on column, in 20% of solvent B, were introduced directly to the mass spectrometer. The red ions represent the precursor ions used for the MRM analyses.