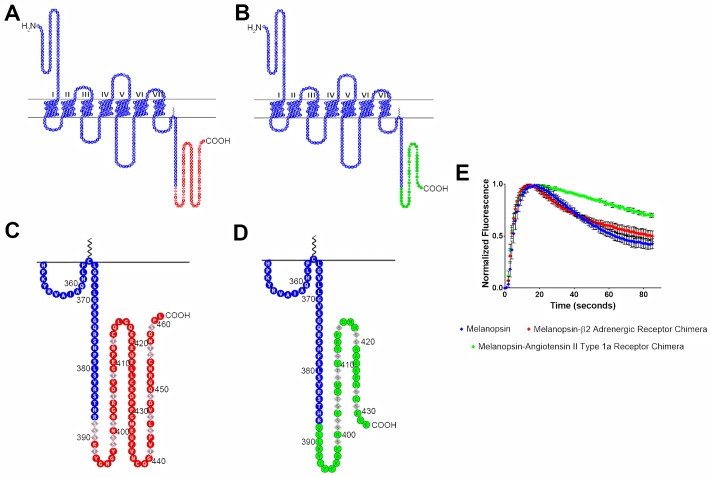
Fig 5. Melanopsin—β2-adrenergic receptor and melanopsin—Angiotensin II type-1a receptor chimeric mutants display different deactivation kinetics.
In both chimeric constructs, melanopsin was truncated at residue 387, which is right before P-II. (A) 2D schematic of melanopsin-β2-adrenergic receptor chimera, melanopsin residues are represented in blue and β2AR residues are represented in red. Phosphorylation sites on β2AR are represented by diamond shaped residues. (B) 2D schematic of melanopsin-angiotensin II type-1a receptor chimera, melanopsin residues are represented in blue and ATII1aR residues are represented in green. Phosphorylation sites are represented by diamond shaped residues. (C & D) Zoomed in schematics of chimeric C-termini of melanopsin-β2-adrenergic receptor chimera and melanopsin-angiotensin II type-1a receptor chimera. (E) Calcium imaging of chimeric mutants, error bars represent the S.D. of the transfection.