Fig. 1.
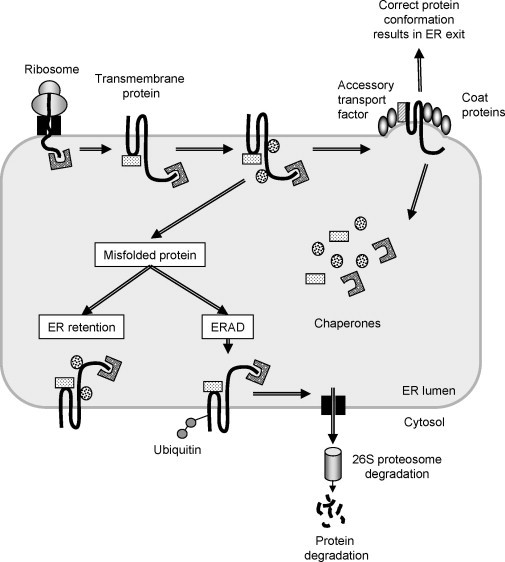
Quality control of protein assembly within the endoplasmic reticulum. Proteins destined for the secretory pathway (this example shows a transmembrane protein) are cotranslationally translocated from the ribosome into the lumen of the endoplasmic reticulum (ER) through a portal referred to as the Sec61 translocon. As the newly synthesized protein enters the ER, quality control mechanisms in the form of protein chaperones bind to it and fold it to its correct conformation. Further processing occurs through interactions with other chaperones before the successfully folded protein is loaded into COPII‐coated vesicles and shuttled from the ER to the Golgi apparatus. However, if the protein carries a mutation that causes it to take on an aberrant conformation the ER chaperones will trigger a misfolded protein response. This has two outcomes: either the chaperones will remain bound to the misfolded protein, preventing its escape from the organelle (ER retention), or the protein will be ubiquitinated and retrotranslocated through the Sec61 complex for proteasomal degradation in the cell cytoplasm. A number of human genetic diseases are a result of key proteins failing to traffic through the secretory pathway and as a consequence are retained or degraded in this manner.
