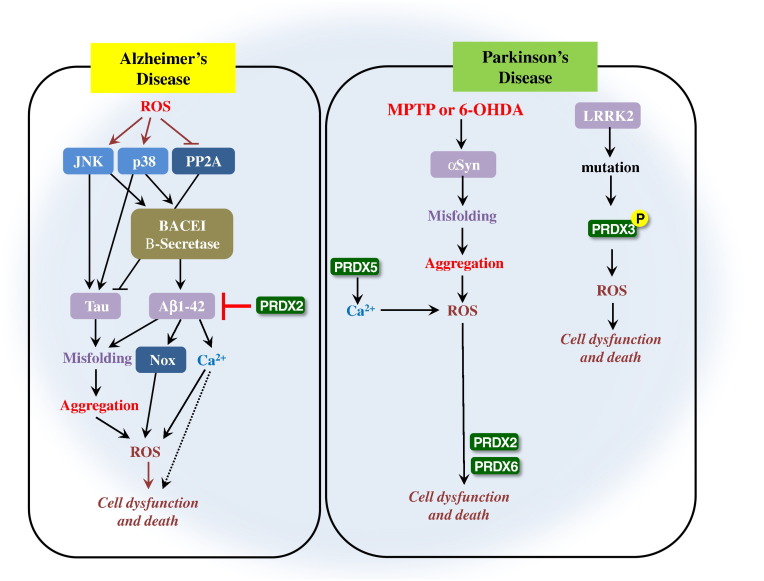
Fig. 5.
Possible mechanisms of PRDXs related with neurodegenerative disease. ROS mediate neurotoxicity of AD through modifying the hallmark protein by oxidation. In AD, ROS could also activate c-Jun N-terminal kinases (JNK) and p38, and deactivate protein phosphatase 2A (PP2A). JNK and p38 promote the expression of Tau, which is inhibited by PP2A. The activation of JNK and p38 further stimulate APP cleaving enzyme 1 (BACE1), causing Aβ1-42 accumulation, which leads to activation of NADPH oxidase (Nox) to produce additional O2, and results in Ca2 + influx to elicit excitatory neurotoxicity. PRDX2 inhibits the generation and accumulation of Aβ1-42, and protects the brain from AD neurotoxicity. PRDX6 accelerates cell dysfunction and cell death proceeded from ROS generation. In PD, the α-Syn is aggregated and generates ROS that also generated from Ca2 + accumulation. PRDX5 induces the Ca2 + accumulation that can cause ROS generation. PRDX2 and PRDX5 mediate the neurotoxicity through induction of cell dysfunction and cell death from ROS accumulation. PD is also generated from LRRK2 mutation. This mutation causes PRDX3 phosphorylation, after that ROS accumulates, resulting in cellular dysfunction and cellular death.