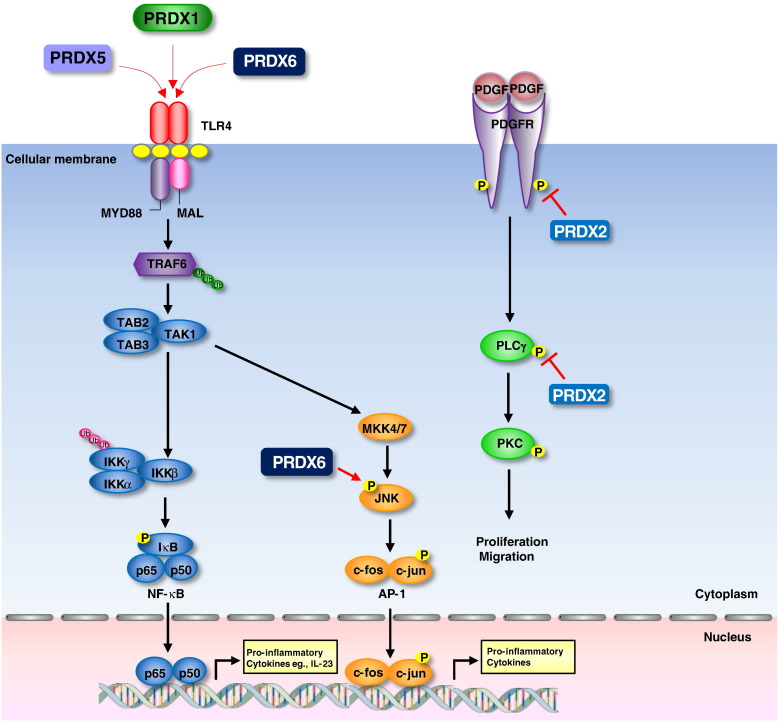
Fig. 6.
Possible mechanisms of PRDXs related with inflammatory disease. PRDX1 can induce activation of NF-κB through TLR4. PRDX1 is secreted from cells under mild oxidative stress and binds with TLR4 which results in induced NF-κB activation that is important for the expression of inflammatory proteins. Recombinant PRDX1 supplemented to the culture medium induced secretion of TNF-α and IL-6 from mouse thioglycolate-elicited peritoneal macrophages and BMDM. This effect of PRDX1 is quite similar to that of LPS as it required membrane proteins CD14 and MD2 and was mediated by the TLR4-MyD88 signaling leading to the activation of NF-κB. It was speculated that PRDXs released from necrotic cells induce NF-κB activation and the production of IL-23. PRDX2 is a negative regulator of PDGF signalling. PRDX2 suppresses activation of PDGF receptor (PDGFR) and phospholipase Cγ1, and subsequently decreases cell proliferation and migration in response to PDGF. Also, PRDX2 is recruited to PDGFR upon PDGF stimulation, and suppresses protein tyrosine phosphatase inactivation. PRDX5 and PRDX6 are TLR4-dependent inducers of infiltrating macrophage activation and the subsequent production of inflammatory mediators from invading T cells in the ischemic brain. Overexpression of PRDX6 promotes development of RA through activation of NF-κB/AP-1 coupled with JNK pathway in the CAIA and AIA-induced arthritis development model.