Fig. 1.
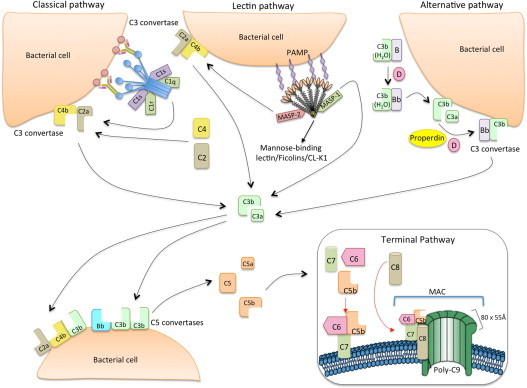
The classical, lectin and alternative pathways of complement activation. The classical pathway is initiated via binding of C1 complex (which consists of C1q, C1r and C1s molecules) through its recognition molecule C1q to target molecules on the surface of pathogens. Subsequently, C1s cleaves C4, which binds covalently to the pathogen surface, and then cleaves C2, leading to the formation of C4b2a complex, the C3 convertase of the classical pathway. Activation of the lectin pathway occurs through binding of mannose-binding lectin (MBL) oligomers, ficolin oligomers or collectin heteromers (CL-K1 + CL-L1), complexed with MBL-associated serine proteases 1 and 2 homodimers (MASP-1 and MASP-2, respectively), to various carbohydrates or acetylated groups on the surface of pathogens (PAMPs: pathogen associated molecular patterns). Like C1s, MASP-2 leads to the formation of the C3 convertase, C4b2a, but its activation is dependent on MASP-1. MASP-1 also cleaves C2. Activation of the alternative pathway depends on spontaneous low-grade hydrolysis of C3 in plasma leading to the formation of C3b. This C3b binds Factor B (homologous to C2) to form a C3bB complex. The cleavage of Factor B by Factor D form the alternative pathway C3 convertase, C3bBb. Properdin stabilizes this complex. The C3 convertases cleave C3 to C3b, which bind covalently next to the site of complement activation (opsonization). This amplifies the cascade and mediates phagocytosis, as well as adaptive immune responses. The addition of additional C3b molecules to the C3 convertase forms C5 convertases (C3bBbC3b for the alternative pathway or C4bC2aC3b for both classical and lectin pathways). This C3b acts as a binding site for C5 and initiate the assembly of the membrane-attack complex (MAC) by cleavage of C5–C5a and C5b. Whereas C5a acts as a potent anaphylatoxin, C5b forms a complex with C6 and C7, which is inserted in the cell membrane. Thereafter, C8 and 10 – 18 C9 molecules (80 × 55 Å each) bind to this complex, resulting in a fully functional MAC (C5b-9). The three pathways converge to this common terminal pathway, culminating with cell lysis and death (Abbas et al., 2012).
