Fig. 1.
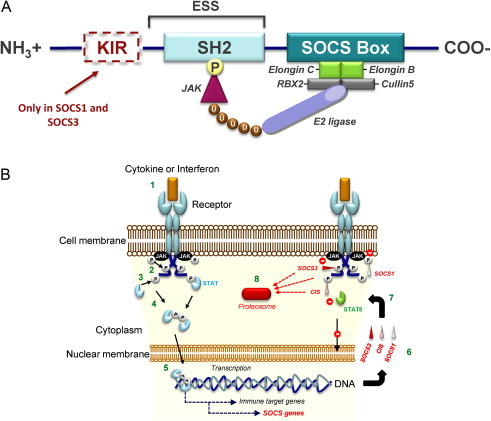
(A) Structure of SOCS proteins. All SOCS proteins have (i) a central SH2 domain, (ii) an amino-terminal end domain of variable length including an extended SH2 sub-domain (ESS) and (iii) a carboxy-terminal SOCS box. SOCS1 and SOCS3 contain an additional amino-terminal kinase inhibitory region known as KIR. The SH2 domain of each SOCS determines its target-specificity, through binding phosphorylated (P) tyrosine residues that are specific to each substrate, such as JAK proteins. The SOCS box interacts with a complex containing elongin B, elongin C, cullin5, RING-box-2 (RBX2), and E2 ligase (also known as E2 ubiquitin-conjugating enzyme). This complex keeps the bound substrate close to the ubiquitinating machinery, thus facilitating its ubiquitination (U) and driving it towards proteosomal degradation. The KIR domain functions as a pseudosubstrate that inhibits the kinase activity of the SOCS-associated proteins. (B) Mechanism of suppression of the JAK/STAT pathway by SOCS1, SOCS3, and CIS. The cytokine or interferon stimulation of their cell surface receptors (1) activates receptor-associated JAK proteins by their phosphorylation (P). Then, activated JAKs phosphorylate receptor cytoplasmic domains (2). Recruited STATs are consequently activated by JAK phosphorylation (3). This phosphorylation enables their dimerization (4). Dimerized they can enter the nucleus and trigger as transcription factor complex the expression of various target genes including SOCS genes (5). Various SOCS proteins such as SOCS1, SOCS3 and CIS are produced (6). They can (7), as SOCS1 but also SOCS3, inhibit the JAK activity with their kinase inhibitory region. They can also, as SOCS3, compete with recruited STAT proteins for shared phosphotyrosine residues or specifically, as CIS, bind the phosphorylated tyrosine residues of cytokine receptors through the SH2 domain consequently masking the STAT5 docking site. Moreover, their SOCS box mediates ubiquitination and degradation of bound receptor components (8). Consecutively to their actions transcription factor complexes cannot anymore form and access the nucleus.
