Fig. 4. In vivo efficacy evaluation of OraEx4.
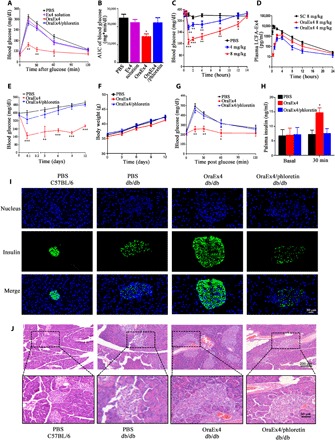
(A) IPGTT after C57BL/6 mice were treated with oral administration of Ex4 solution (4 mg/kg), OraEx4 (4 mg/kg), or OraEx4 (4 mg/kg) together with phloretin (50 mg/kg; n = 6). (B) AUC of blood glucose levels in (A). (C) Blood glucose levels after a single treatment of OraEx4 (4 or 8 mg/kg) in db/db mice (n = 5). (D) Pharmacokinetic profiles of Oral-Ex4 (4 and 8 mg/kg), compared with subcutaneous (SC) injection of LCFA-Ex4 (n = 5). (E) Long-term blood glucose regulation by daily gavage of OraEx4 (8 mg/kg; n = 5). (F) Body weight changes in db/db mice during treatment with OraEx4 for 12 days (n = 5). (G) IPGTT after treatment with a single dose of OraEx4 (8 mg/kg) in db/db mice (n = 5). (H) Insulin levels before and after OraEx4 treatment in db/db mice (n = 5). (I) Immunofluorescence images on insulin secretion from islets in C57BL/6 and db/db mice. (J) H&E staining of pancreatic islets from posttreatment db/db mice. Images show representative islets from five mice per group. Data are presented as means ± SEM, *P < 0.05, **P < 0.01, ***P < 0.001.
