Fig. 6. Nucleocapsid assembly occurs in liquid droplets and is enhanced by phase separation.
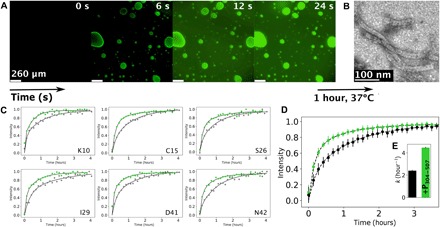
(A) Fluorescence microscopy images showing fluorescently labeled RNA diffusing into droplets preformed by mixing P50N525 (20 μM) and P304–507 (80 μM); RNA of 200 μM (with 10% fluorescently labeled) was added on one side of the coverslip and spontaneously diffused in the sample. (B) Negative-stain electron microscopy of the sample shown in (A), after 1 hour of incubation at 37°C. (C) Kinetic traces of peak intensities corresponding to individual residues of P1–50 in SOFAST 1H-15N HMQC spectra acquired at a resolution of 8.5 min (8 scans, 200 15N increments, and 1024 1H), following addition of OH-A6-OH RNA to P50N525 (gray) and under phase-separating conditions with P304–507 (green) at 293 K. Data were fitted with a biexponential function as previously described (13). (D) Averaged intensities for residues E4, K10, N11, G12, E14, C15, I16, G25, S26, L27, I29, A32, M33, A35, D41, N42, G44, and Q45 of P1–50 in P50N525 (gray) and under phase-separating conditions with P304–507 (green). Dots with SD represent experimental data, and lines represent biexponential fit. (E) Rate of assembly of nucleocapsids as measured by the appearance of resonances from P50 when assembled using P50N525 (gray) and when using P50N525 + P304–507 (green) having formed droplets. The concentration of P50N525 and RNA was constant in both cases.
