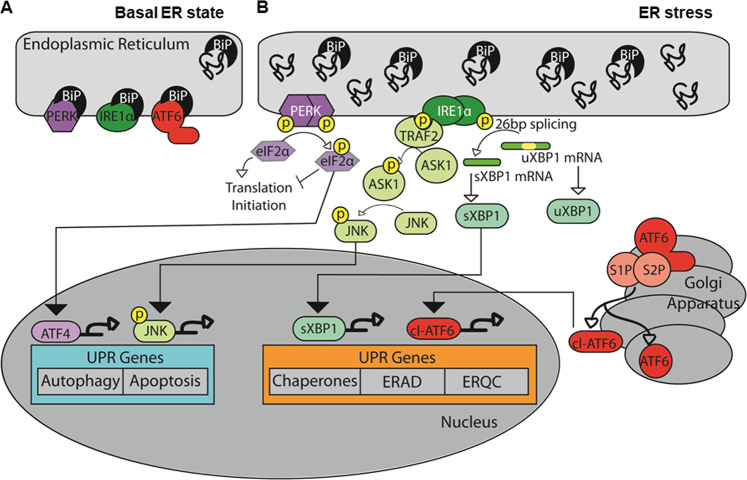
Fig. 1.
Signaling pathways from three ER transmembrane stress sensors during unfolded protein response (UPR) in schizophrenia. a ER stress stimulates the activation of the three ER transmembrane stress sensors, protein kinase RNA-like ER kinase (PERK), activating transcription factor-6 (ATF6), and inositol requiring enzyme 1α (IRE1α), that are involved in the unfolded protein response (UPR). a Under basal, unstressed conditions, the UPR sensors are present within the ER membrane. The heat shock protein BiP (Grp78) binds these receptors and prevents them from forming homodimers and from translocating out of the cell. b After dissociation of BiP, ATF6 translocates to the Golgi where it is cleaved and trafficked to the nucleus, while PERK and IRE1α form homodimers and autophosphorylate. Activation of the PERK pathway leads to phosphorylation of eukaryotic translation initiation factor 2α (eIF2α) and increased expression of activating transcription factor 4 (ATF4), while activation of the IRE1α pathway leads to unconventional mRNA splicing of the transcription factor x-box binding protein 1 (XBP1). In addition, IRE1α activation recruits TNF receptor associated factor 2 (TRAF2) and results in phosphorylation of apoptosis signal-regulating kinase 1 (ASK1), which then phosphorylates the transcription factor c-Jun N-terminal kinase (JNK). ATF6, ATF4, and spliced XBP1 (sXBP1) function as transcription factors of UPR target genes, such as ER chaperones, the ER quality control system (ERQC) and ER-associated degradation (ERAD)