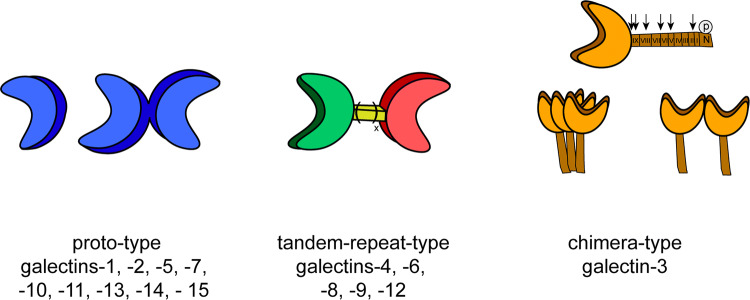
Fig. 1.
Overview of the classification of the three types of modular architecture of vertebrate galectins. Proto-type galectins contain a single carbohydrate recognition domain (CRD) and are able to form monomeric or homodimeric structures. Tandem-repeat-type galectins have two distinct CRDs and are covalently associated via a linker peptide with natural variation of linker length by alternative splicing; chimera-type galectin, i.e., galectin-3 harbors one CRD and a non-lectin domain which consists of an N-terminal region and nine collagen-like repeat units that are substrates for matrix metalloproteinases (MMP-2/-7/-9/-13) and PSA-mediated cleavage at different positions shown by arrows. The N-terminal region functions as a site for serine phosphorylation. Galectin-3 is monomeric in solution in the absence of a ligand and can form aggregates in contact to oligo- or polyvalent ligands via the N-terminal tail, the CRD, or both