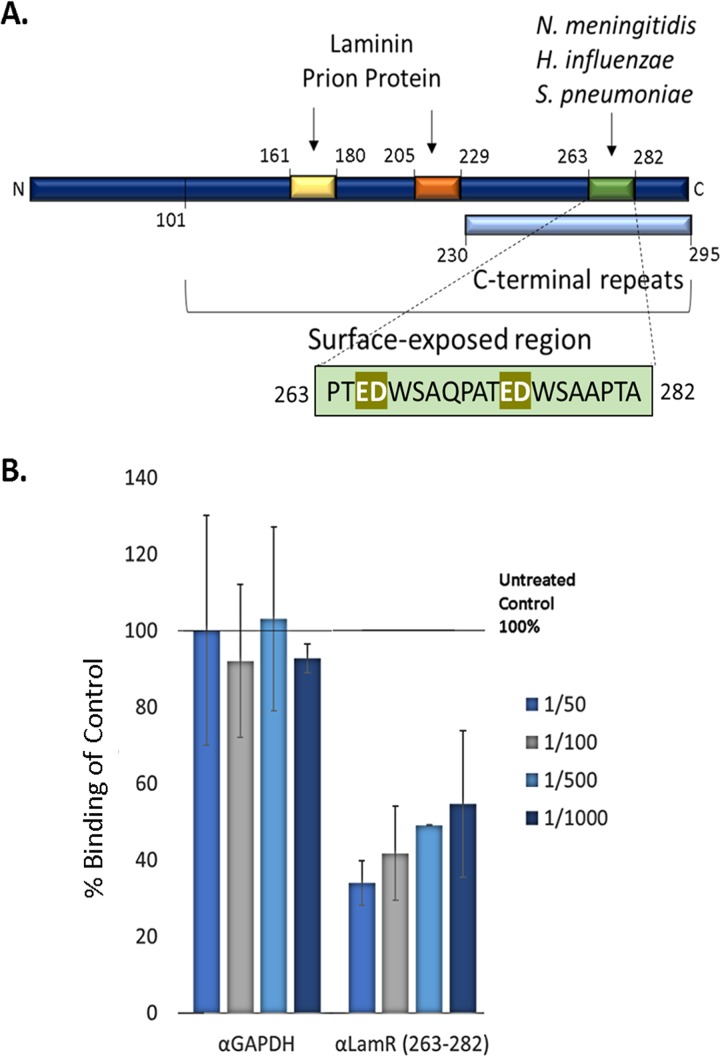
FIG 7.
T. pallidum interacts with LamR (aa 263–282) on brain endothelial cell surfaces. (A) Schematic illustration of characterized interaction regions of LamR (51, 71, 72, 74, 75). Elements include the predicted surface-exposed region (aa 101–295), C-terminal repeats (light blue box, aa 230–295), binding sites for laminin and the prion protein, including the peptide G region (yellow box; aa 161–180) and direct binding region (orange box; aa 205–229) and the neuroinvasive bacterial pathogen binding site (green box; aa 263–282). (B) T. pallidum attachment to brain endothelial cells (hCMEC/d3) was evaluated using immunofluorescence detection of adherent treponemes (FlaA; red) and hCMEC/d3 nuclei (DAPI; blue) at ×200 magnification from five FOV in duplicate from two independent experiments. Inhibition of T. pallidum attachment to hCMEC/d3 cells by antibodies was quantified as a percentage of the untreated no-antibody control (set at 100%). Results are presented as means ± SEM, and statistical analysis was performed by two-way ANOVA comparing T. pallidum binding to endothelial cells following hCMEC/d3 preincubation with anti-GAPDH (α-GAPDH) versus anti-LamR (aa 263–282) where P = 0.0037.