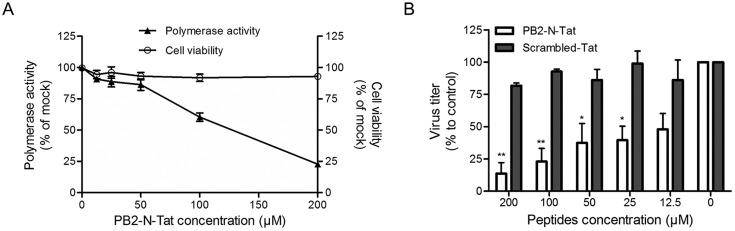
Fig. 1.
The PB2-Nter derived peptide inhibited influenza polymerase activity and virus replication. (A) The inhibitory effect of peptide PB2-N-Tat on viral polymerase activity (left y-axis) was tested by a mini-replicon assay, while its cytotoxicity to 293T cells (right y-axis) was determined by an MTT assay. In the mini-replicon assay, indicated concentrations of PB2-N-Tat were added at 5 h post-transfection. Luminescence and fluorescence were determined at 24 h post-transfection, respectively. In the MTT assay, PB2-N-Tat with various concentrations was incubated with 293T cells for 24 h before addition of MTT substrate. The experiments were carried out in triplicate and repeated twice. Data are presented as mean values ± SD. (B) The antiviral effect of PB2-N-Tat was evaluated in MDCK cells using a multi-cycle virus growth assay. Cells were infected by A(H1N1)pdm09 influenza virus at MOI of 0.002. After virus internalization, the inoculum was removed, washed and replaced by culture medium containing peptides with indicated concentrations. A scrambled Tat-peptide was included as a negative control. Virus titers in the cell supernatant were collected at 24 h post-infection and determined by plaque assays. Results are presented as mean values + SD of two independent experiments. *p < 0.05, **p < 0.01 compared with the scrambled-Tat-treated group by unpaired t-test.