Abstract
Introduction:
Cross-infection or contamination are the major threats related to any medical profession. Microorganisms present in the dental clinic can cause cross-infection to the dentist, auxiliary staff and even towards the patients.
Aims:
The study was conducted to assess the level of atmospheric microbial contamination and composition of aerosols before, during and after dental treatment procedures in four clinical settings.
Methods and Materials:
The present study was conducted over a two-week period in a private dental college setting. An equal number of culture medium plates (blood agar) were placed 30 min prior to the initiation of work sessions in the selected area and 1 h after the working session began and after 2 h of cessation of the working period. After the collection of samples, the culture medium plates were incubated aerobically at 37°C in an incubator for 48 h. The number of colonies was expressed as colonies per media plate. After counting the colonies bacterial cell morphology was determined by a microscopic examination using a Reichert-Jung Series 150 light microscope.
Statistical Analysis:
Statistical analysis such as ANOVA test for mean values and post hock was done using statistical package for social sciences (SPSS).
Results:
It shows that colony count increased after the working session and which reduced by itself once the working session was concluded which was significant (P < 0.001). The highest increase in the mean colony count was found in the department of periodontology during the treatment sessions. In the blood agar plates, the S. epidermidis was found maximum 62%, micrococcus was 22%, diphtheroid was 10%, fungi 4% and the least S. aureus 2%.
Conclusion:
This study demonstrates that aerosols increase during and after work sessions and, therefore, increases the chance for infectious agent transmission in clinical settings.
Keywords: Bacterial aerosols, clinical dentistry, cross-infection, microbial contamination
Introduction
Infection control is considered one of the major apprehensions of the dental fraternity. Infections can be transmitted through saliva or blood, direct or indirect contact, through the aerosols produced during the treatment performed, or even the contaminated instruments and equipment.[1,2]
Bioaerosols are an important contemplation for infection control and should be given distinct consideration in occupational health, as these may consist and transmit hazardous organisms, allergens, and other toxic substances. This suggests cross-contamination may happen through the aerosols in dental clinics also. Although the existence of these microbiological aerosols has been known since long their role in dentistry has been investigated recently.[1,3]
In dental clinics, contaminated air may contain particles derived from saliva, blood, dental plaque, calculus, tooth debris, or dental filling materials and may epitomize an important potential source of infection.[4,5] These infections can cause serious health issues for all the people working in the dental clinic and must be considered as an occupational health hazard.
Microorganisms can be suspended in air and are responsible for causing various infections such as the common cold, pneumonia, tuberculosis, herpes, hepatitis B, and AIDS as well as skin and eye infections. The spread of infections depends on various factors which include humidity, temperature, particle size, and ventilation.[6,7,8] Most of the dental aerosols generated from the high-speed hand piece, ultrasonic scaler or water/air syringe have a diameter of 50 μm or less and are mostly concentrated within 2 ft of the patient's mouth.[9] However, the ability of these aerosols to produce infections relies on the quantity and pathogenicity of the invading microorganisms, as well as the immune capacity of the patient.
In a study by Grenier in 1995 the quantity of bacterial air contamination during treatments in both closed dental operatory and multi chair dental clinic was assessed. He concluded that bacterial count increases by the dental treatments performed in both operatories.[10]
According to the center for disease control (CDC) guidelines for infection control in dental healthcare settings (2003),[11] preventive measures to control dental office air contamination include universal precautions, which consist of dental staff protective equipment (Gown, Mask, Gloves, Eyeglasses), preprocedural patient mouth rinsing with antimicrobial products like chlorhexidine gluconate,[12] operatory isolation (rubber dam), vacuum and electrostatic extraction of aerosols during dental procedures, air circulation methods (ventilation and air-conditioning systems), air filtration systems for solid particles and mercury, disinfectants or organic compounds vapours, ultraviolet lamps, and microbial controls for instrument and surfaces.[13,14,15]
Aim
The aim of the study was to assess the level of atmospheric microbial contamination as well as composition of aerosols before, during and after dental treatment procedures in clinical settings, to quantify the risk of staff and patient exposure to aerosolized microbial pathogens and motivate the implementation of protective methods for making it a safe place to live in.
Material and Methods
The present study was conducted in a private college of dental science settings. Four sites were selected within the dental college:
Department of Periodontics
Department of Prosthodontics
Department of Conservative and Endodontics
Sterilization chamber of Oral Surgery Department
An equal number of culture medium plates (blood agar) were placed 30 min prior to the initiation of work sessions in the selected area. Using a method similar to Johnston et al.,[16] 20-minute exposure of blood agar culture medium plate was used to collect the airborne bacteria.
The same procedure was repeated 1 h after the working session began. In the dental clinic, culture medium plates were placed 2–3 ft away from the patient's mouth since this is the area where bacterial aerosol concentration is at its highest, as reported by Micik et al.[9]
The third set of culture medium plate was placed after 2 h of the working period.
These procedures were performed on Saturday, Monday, and Wednesday over a 2-week period and the number of plates used and the position of the plates were the same for the before, during, and after analysis period.
After the collection of samples, the culture medium plates were incubated aerobically at 37°C in an incubator for 48 h. After that bacterial colony counting was done by a microbiologist. The number of colonies was expressed as colonies per media plate (c/plate). Microbiologist was unaware of the culture medium plates, time of exposure, or location.
After counting the colonies bacterial cell morphology was determined by a microscopic examination using a Reichert-Jung series 150 light microscope.
Data collected were statistically analyzed using the statistical package for social sciences (SPSS) version 20.0. One way analysis of variance (ANOVA) was performed to test the overall differences between means. A paired t-test was used to test the differences between specific means.
Results
Table 1 shows the comparison of the mean colony count in the various departments included in the study, before, during, and after the working sessions. It was found that colony count increased after the working session which reduced by itself once the working session was ceased and was found significant (P < 0.001).
Table 1.
Comparison of mean colony count in different departments (before, during, and after the treatment
| Department | Before (1) (mean±SD) | During (2) (mean±SD) | After (3) (mean±SD) | ANOVA F/P | Post Hoc |
|---|---|---|---|---|---|
| Periodontics | 29.17±9.70 | 146.67±34.30 | 47.50±5.24 | 55.401 <0.001 (S) | 2>1,3 |
| Conservative | 30.83±11.58 | 93.33±10.80 | 37.5±8.80 | 64.581 <0.001 (S) | 2>1,3 |
| Prosthodontic | 28.33±4.082 | 70.00±7.75 | 37.5±5.24 | 82.840 <0.001 (S) | 2>1,3 3>1 |
S=Significant
The mean colony counts for various departments before the treatment are compared in Figure 1. This clearly indicates the highest count which was seen in the department of conservative dentistry.
Figure 1.
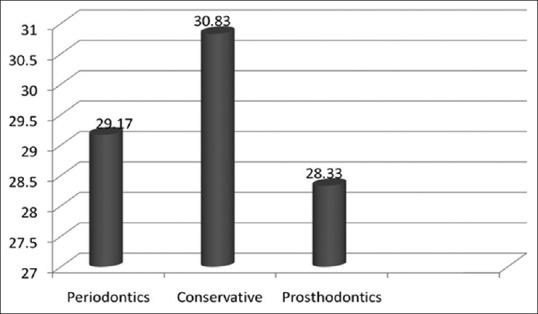
Comparison of mean colony count for the departments before treatment P-value>0.889 (NS)
Figure 2 represents the mean colony count during the treatment sessions in all the three departments which shows the highest increase in the colony count in the department of periodontology.
Figure 2.
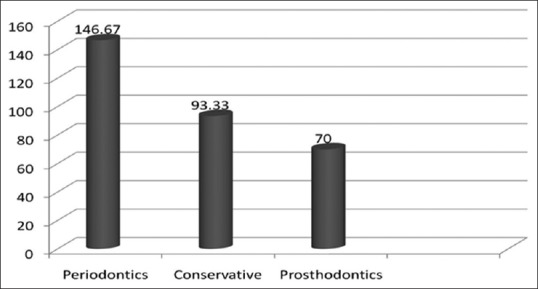
Comparison of mean colony count for the departments during the treatment P-value < 0.001(S)
Similarly, Figure 3 reveals the reduction in the colony count once the working session ends which was also significant (P < 0.001).
Figure 3.
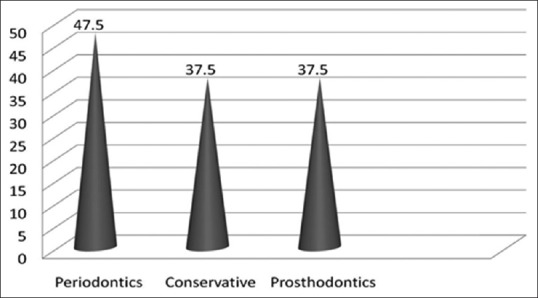
Comparison of mean colony count for the departments after the treatment P-value < 0.05 (S)
Figure 4 reveals a comparison of the mean colony count before and after the treatment session of the sterilization room where it was found that there was an obvious increase in the CFUs after the working session.
Figure 4.
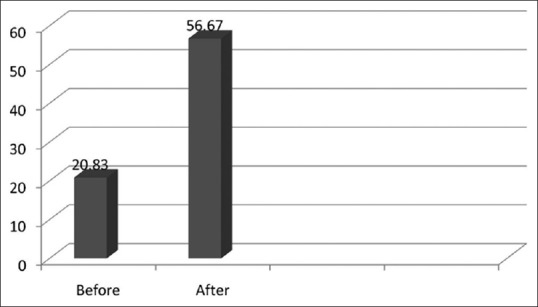
Comparison of mean colony count for the sterilization room of the oral surgery department (before and after the treatment) P-value< 0.01 (S)
In Figure 5 reveals Pie diagram with the percentage of various microorganisms found in the blood agar plates, in which the S. epidermidis was found 62%, micrococcus was 22%, diphtheroid was 10%, fungi 4% and the least S. aureus 2%.
Figure 5.
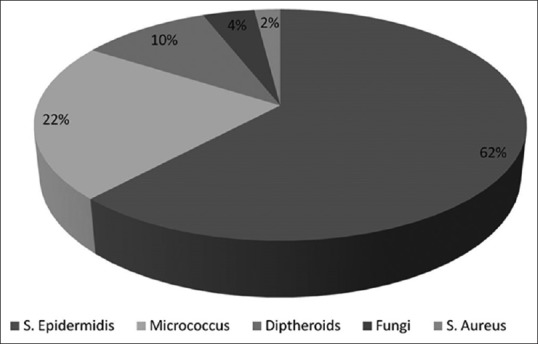
Percentage of microorganism colony composition
Discussion
The present study was conducted in four different clinical settings of a private dental college situated in Madhya Pradesh, India. A safe environment is an important consideration for all dental personnel and patients. The present study depicts the kind of antimicrobial exposure any health professional will be exposed to and that proper care needs to be taken of the patient, doctor, and auxiliary staff in the dental settings.
About 5% of sheep blood agar media plates were used because it was valid for collecting airborne microorganism and for cultivation of fastidious pathogenic microorganisms.[10] It is a nonselective, enriched medium and it is used for general purpose; it promotes the growth of microbes such as those sampled from air.[10,17] It is likely that the actual microbial content in the specified area was much higher than reported here, as the culture medium and growth condition used did not allow the identification of all types of organisms including viruses and other organisms requiring specialized medium.
In the present study level of bacterial air, contamination arose during dental treatment and laboratory procedures which were higher than what was seen before and after the treatment. Larato et al.[18] have observed similar patterns of microbial air contamination before, during, and after dental treatment in the closed operatory.
The subsequent decrease of bacterial air contamination was noticed 30 min after the end of the working period. This is in agreement with results reported by Grenier et al.,[10] Larato et al.,[18] and Travaglini et al.[19] who suggested that the decrease in level could be referred to as the rapid settling of bacterial laden particles.
Lorato and others[18] and Miller et al.[20] reported that when droplets containing organisms from the mouth are forced into the air, they react in two ways. Heavy droplets fall to the floor and become part of floor dust. Aerosol particles light in weight remain suspended in the air for an extended period and may eventually reach the respiratory passages of anyone exposed to the residue.
Dental operators and dental assistants should always wear mouth masks, gloves, eyeglasses, lateral protective shields, and head caps. A group of researchers has also recommended patients to rinse their mouth with an antiseptic solution (chlorhexidine gluconate) for the reduction of the microbial contents of aerosols prior to dental surgery.[21] However, adherence to strict infection control procedures may increase the cost of treatment, which can affect the pattern of dental practice.
The studies conducted by Mills[22] has shown that there is a microbial growth when the layer from the turbine handpiece was sent for the culture after treatment. When evaluating the bacterial contamination composition, the main components were S. epidermidis, micrococcus, diphtheroid, S. aureus, and fungi.
S. epidermidis is located on the skin and spreads through contact. It is an infection which arises only when an opportunity arises, hence it is considered an opportunistic pathogen. It is, however, the most common cause of infection in patients with implanted prosthetic devices.[23] such as heart valves, artificial joints, and catheter-related sepsis.
Micrococci, found in abundance on the lingual surface within the oral cavity, often with a predilection for the tongue surface, is similar to Staphylococci. Diphtheroids, normally inhabiting the skin and conjunctiva, are occasionally opportunistic pathogens in compromised patients.
S. aureus normally inhabits human skin and mucous membranes, especially the anterior nares and the perineum and is usually transmitted via the hands. It is a common cause of a variety of diseases including wound infections, abscesses, septicemia, osteomyelitis, endocarditis, and a number of respiratory infections.
It is important to note that Staphylococcus species demonstrate resistance (multi-resistance) to a number of drugs including penicillin and methicillin. It is frequently found as contaminating bacteria in clinical specimens and may cause infections like pneumonia and eye infection and are always present in the hospital and clinical environments.[24]
Staphylococcus species were found in the indoor air of dental school and the active role of dentistry operations in microbial contamination of various parts of the dental school with or without direct involvement with the dental operation was noticed. This could be due to the frequent use of devices with propelling force such as high-speed dental drills combined with water spray, which can generate numerous airborne infectious microbial agents. Transmission of infectious diseases associated with indoor environment of dental clinics could be acquired by dental staff and patients by airborne transmission.[25.26]
This research demonstrates the need for the management of the possible risk of infective hazards among dental personnel in an Indian dental school. Therefore, formal and informal educational programs along with performing periodic checks on environmental contamination are recommended to improve the quality of the dental school environment.
A Study conducted by Fine et al.[27] as also many other studies, has proved that preprocedural oral rinsing with an antiseptic mouthwash significantly reduces the viable microbial content of bioaerosols generated during dental operative procedures. They concluded that this pre-procedural rinsing may have a potential role in reducing the risk of cross-contamination with an infectious agent in the dental operatory[27,28] Previous research demonstrated that rinsing with antiseptic mouth wash produced a 94.1% reduction in recoverable CFUs compared to nonrinse control.
In dental clinics, several infectious agents could be acquired by dental staff and patients by airborne transmission, in addition, dental aerosols containing opportunistic pathogens should also be considered hazardous for immunosuppressed patients, who could develop serious infections. Laminar unidirectional airflow, air ventilation, and air filtration could also be beneficial in dental environment and should be considered.
In the present study, the mean CFU in the department of periodontics, endodontics, and prosthodontics during the working hours were 146.67, 93.33, and 70 CFU, respectively while a study published in 1995 reported a level of contamination of 216 CFU/M3 for ultrasonic scaling treatment and 75 CFU/M3 for operative treatment[10] and a more recent study by Mansour et al.[1] found 120–180 CFU/M3 in the air in dental surgeries. The ultrasonic scaling was obviously associated with increased air contamination levels confirming the results reported by several other studies showing that this procedure is one of the greatest producers of airborne contaminants in dentistry.[29,30]
Two recent studies have highlighted the spread of infection through the air resulting from the most intensive aerosol and splatter emission that occurs from an ultrasonic scaler tip and bur on a high-speed hand piece.[31,32]
A study published in 2000 reported that microbial aerosol peak concentration in the dental treatment room was associated with scaling procedure (47% of procedure giving rise to a peak) and to a lesser extent by cavity preparation (11%).[33]
Results of the present study must be used for increasing awareness and quantifying the risk of staff and patient exposure to aerosolized microbial pathogens in general dental office, which must be controlled by efficient preventive measures. These include protective clothing and equipment for the staff, pr-procedural patient oral rinses, high volume evacuators, ventilation, and air filtration.[34,35]
Procedure rooms should be periodically disinfected by fumigation. Preprocedural use of mouth rinses has also been shown to be effective in reducing aerosol contamination. Further bioaerosol monitoring is recommended to track and control hospital-associated infections as well as for the purpose of surveillance for infection control.[36]
This study is of prime importance towards spreading awareness regarding the ill-effects of aerosols in dental clinical settings which can be a great occupational hazard in the working area for the doctor and auxiliary staff. This could even lead to cross-infection among other patients and the caretakers visiting along with the patients.
Conclusion
This study demonstrates that aerosols increase during and after work sessions and, therefore, increase the chance for infectious agent transmission. Preventive measures should be instituted to reduce or disrupt aerosols as a transmission route in the multi chair dental clinic, sterilization center, and prosthetic laboratory. This can lead to serious health issues not only for the people working there including the auxiliary staff and doctors but for the patients and for people accompanying the patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Mansour RA, Ali G, Mohammad RM, Negar FN. Airborn microbial contamination of dental units. Tanaffos. 2008;7:54–7. [Google Scholar]
- 2.Merchant VA. Herpesvirus and other microorganisms of the concern to dentistry. Dent Clin North Am. 1991;35:283–98. [PubMed] [Google Scholar]
- 3.Al Maghlouth A, Al Yousef Y, Al Bagieh N. Qualitative and quantitat ive analysis of bacterial aerosols. J Contemp Dent Pract. 2004;4:91–100. [PubMed] [Google Scholar]
- 4.Harrel SK, Molinari J. Aerosols and splatter in dentistry. JA DA. 2004;135:429–37. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lu DP, Zambito RF. Aerosols and cross infection in dental practice- A historic view. Gen Dent. 1981;29:136–42. [PubMed] [Google Scholar]
- 6.Burge HA. Indoor sources for airborn microbes. In: Gammage RB, Kaye SV, editors. Indoor Air and Human Health. Chelsea, Mich: Lewis Publishers, Inc; 1985. pp. 139–48. [Google Scholar]
- 7.Kraidman G. The microbiology of airborn contamination and air sampling. Drug Cosm Ind J. 1975;3:40–3. [Google Scholar]
- 8.Walter CW. Prevention and control of airborn infection in hospitals. Ann NY Acad Sci. 1980;353:312–20. doi: 10.1111/j.1749-6632.1980.tb18935.x. [DOI] [PubMed] [Google Scholar]
- 9.Micik RE, Miller RL, Mazzaerella AM, Ryge G. Studies on dental aerobiology. I. Bacterial aerosols generated during dental procedures. J Dent Res. 1969;48:49–56. doi: 10.1177/00220345690480012401. [DOI] [PubMed] [Google Scholar]
- 10.Grenier D. Qualitative analysis of bacterial aerosols in two different dental clinic environments. Appl Environ Microbiol. 1995;61:3165–8. doi: 10.1128/aem.61.8.3165-3168.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sotiriou M, Ferguson SF, Davey M, Wolfson JM, Demokritou P, Lawrence J, et al. Measurmement of particle concentration in dental office. Env Monitor Asses. 2008;137:351–61. doi: 10.1007/s10661-007-9770-7. [DOI] [PubMed] [Google Scholar]
- 12.Fine DH, Furgang D, Korik I. Reduction of viable bacteria in dental aerosols by preprocedural rinsing with an antiseptic mouth rinse. Am J Dent. 1993;6:219–21. [PubMed] [Google Scholar]
- 13.Jain M, Sawla L, Mathur A, Nihlani T, Ayair U, Prabu D, et al. Knowledge, attitude and practice towards droplet and airborne isolation precautions among dental health care professionals in Udaipur, Rajasthan, India. Med Oral Patol Oral Cir Bucal J. 2010;15:957–61. doi: 10.4317/medoral.15.e957. [DOI] [PubMed] [Google Scholar]
- 14.Shivakumar KM, Prashant GM, Madhu Shankari GS, Subba Reddy VV, Chandu GN. Assessment of atmospheric microbial contamination in a mobile dental unit. Ind J Dent Res. 2007;18:177–80. doi: 10.4103/0970-9290.35828. [DOI] [PubMed] [Google Scholar]
- 15.Barnes JB, Harrel SK, RHidalgo F. Blood contamination of the aerosols produced by the in vivo use of ultrasonic scalers. J Periodontol. 1998;69:434–8. doi: 10.1902/jop.1998.69.4.434. [DOI] [PubMed] [Google Scholar]
- 16.Johnston JR, Butchart AM, Kgamphe SJ. A comparison of sampling methods for airborne bacteria. Environ Res. 1978;16:279–84. doi: 10.1016/0013-9351(78)90162-7. [DOI] [PubMed] [Google Scholar]
- 17.King TB, Muzzin KB, Berry CW, Anders LM. The effectiveness of an aerosol reduction device for ultrasonic scalers. J Periodontol. 1997;68:45–9. doi: 10.1902/jop.1997.68.1.45. [DOI] [PubMed] [Google Scholar]
- 18.Larato DC, Ruskin PF, Martin A, Delanko R. Effect of dental air turbine drill on the bacterial count in air. J Prosthet Dent. 1966;16:758–65. [Google Scholar]
- 19.Travaglini EA, Larato DC, Martin A. Dissemination of organism bearing droplets by high speed dental drills. J Prosthet Dent. 1966;16:132–39. [Google Scholar]
- 20.Miller RL, Burton WE, Spore RW. Aerosols produced by dental instrumentation. Proc First Intern Symp Aerobiol. 1963:97–120. [Google Scholar]
- 21.Logothetis DD, Martinez Welles JM. Reducing bacterial aerosol contamination with a chlorhexidine gluconate pre-rinse. J Am Dent Assoc. 1995;126:1634–39. doi: 10.14219/jada.archive.1995.0111. [DOI] [PubMed] [Google Scholar]
- 22.Mills SE, Kuchne JC, Bradley DV. Bacteriological analysis of high speed handpiece turbines. J Am Dent Assoc. 1993;124:59–62. doi: 10.14219/jada.archive.1993.0014. [DOI] [PubMed] [Google Scholar]
- 23.Samaranayake LP, Jones BM. Essential Microbiology for Dentistry. New York: Church II Livingstone; 1996. [Google Scholar]
- 24.Jenesen MM, Wright DN. Introduction to Microbiology for the Health Science. 2nd ed. New Jersey: Prentice Hall International; 1989. [Google Scholar]
- 25.Merchant VA. Herpesviruses and other microorganisms of concern in dentistry. Dent Clin North Am. 1991;35:283–98. [PubMed] [Google Scholar]
- 26.Mori M. Status of viral hepatitis in the world community: Its incidence among dentists and other dental personnel. Int Dent J. 1984;34:115–21. [PubMed] [Google Scholar]
- 27.Fine DH, Mendieta C, Barnett ML, Furgang D, Meyers R, Olshan A, et al. Efficacy of preprocedural rinsing with an antiseptic in reducing viable bacteria in dental clinic. J Periodontol. 1992;63:821–4. doi: 10.1902/jop.1992.63.10.821. [DOI] [PubMed] [Google Scholar]
- 28.Trenter SC, Walmsley AD. Ultrasonic dental scaler: Associated hazards. J Clin Periodontol. 2003;30:95–101. doi: 10.1034/j.1600-051x.2003.00276.x. [DOI] [PubMed] [Google Scholar]
- 29.Harrel SK, Barnes JB, Rivera-Hidalgo F. Aerosol and splatter contamination from the operative site during ultrasonic scaling. JADA. 1998;129:1241–9. doi: 10.14219/jada.archive.1998.0421. [DOI] [PubMed] [Google Scholar]
- 30.Timmerman MF, Menso L, Steinfort J, Van Winkelhoff AJ, Van Der Weijden GA. Atmospheric contamination during ultrasonic scaling. J Clin Periodontol. 2004;3:458–62. doi: 10.1111/j.1600-051X.2004.00511.x. [DOI] [PubMed] [Google Scholar]
- 31.Leggat PA, Kedjarune U. Bacterial aerosols in the dental clinic: A review. Int Den J. 2001;51:39–44. doi: 10.1002/j.1875-595x.2001.tb00816.x. [DOI] [PubMed] [Google Scholar]
- 32.Szymanska J. Dental bioaerosols as an occupational hazard in a dentist's workplace. Annals Agri Envi Med. 2007;14:203–7. [PubMed] [Google Scholar]
- 33.Bennett AM, Fulford MR, Walker JT, Bradshaw DJ, Martin MV, Marsh PD. Microbial aerosol in general dental practice. Br Dent J. 2000;189:664–7. doi: 10.1038/sj.bdj.4800859. [DOI] [PubMed] [Google Scholar]
- 34.Cellini L, Di Campi E, Di Candia M, Chiavaroli G. Quantitative microbial monitoring in a dental office. Public Health. 2001;115:301–5. doi: 10.1038/sj.ph.1900746. [DOI] [PubMed] [Google Scholar]
- 35.Jacks ME. A laboratory comparison of evacuation devices on aerosol reduction. J Dent Hyg. 2002;76:202–6. [PubMed] [Google Scholar]
- 36.Madhuri KR, Girish BRJ. Evaluation of bacterial aerosol contamination during dental procedures. Int J Med Microbiol Trop Dis. 2019;5:23–7. [Google Scholar]


