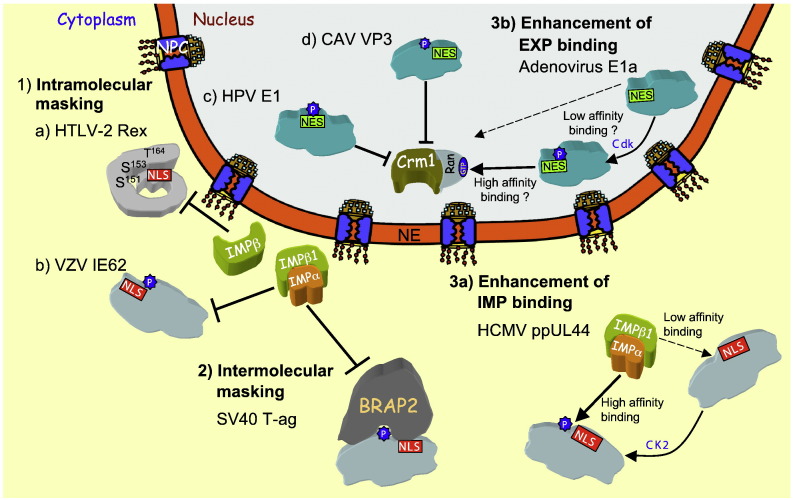
Fig. 3.
Schematic representation of the mechanisms of regulation of IMP/EXP-dependent nuclear transport, as illustrated by examples of viral proteins. In intramolecular masking (1) IMPs/EXPs are prevented from binding the NLS/NES of the cargo by masking of the NLS/NES by sequences within the same protein. This is exemplified by (a) the human T-cell leukaemia virus type 2 (HTLV-2) Rex protein in its inactive p24 form, where specific phosphorylation by CK1/GSK3 at T164 and subsequent phosphorylation at S151/153 are required for IMPβ recognition of the NLS, and (b) the VZV IE62, where phosphorylation at S686 by the VZV kinase ORF66 inhibits nuclear import by impairing recognition by IMPα/β1 [97]. Examples of intramolecular masking in nuclear export are shown for (c) HPV E1, where Cdk mediated phosphorylation of S107 prevents Crm1 binding, resulting in nuclear retention [24], [84], and (d) CAV VP3, where phosphorylation of T108 specifically in cancer cells prevents nuclear export [114], [115]. In intermolecular masking (2), NLSs/NESs are masked from IMPs/EXPs binding by a heterologous protein/molecule. An example in the case of nuclear import is the cytoplasmic protein BRAP2 which, dependent on Cdk phosphorylation of T124, prevents recognition by IMPα/β1 of the SV40 T-ag NLS, a similar mechanism dependent on protein kinase C phosphorylation of T427 applies to HCMV ppUL44 protein (not shown) [78]. Enhanced nuclear import/export can occur through posttranslational modification enhancing NLS/NES recognition by IMP/EXP. An example (3a) is the increase in the affinity of binding of IMPα/β1 to the NLSs of HCMV ppUL44 and SV40 T-ag (not shown) by CK2 phosphorylation (of S413 and S111/112 respectively) leading to enhanced nuclear import [23], [85], [87]. In the case of nuclear export (3b), Cdk1/Cdk2 mediated phosphorylation of S89 enhances recognition of the Adenovirus type 5 E1a NES by Crm1, leading to more efficient nuclear export [111].