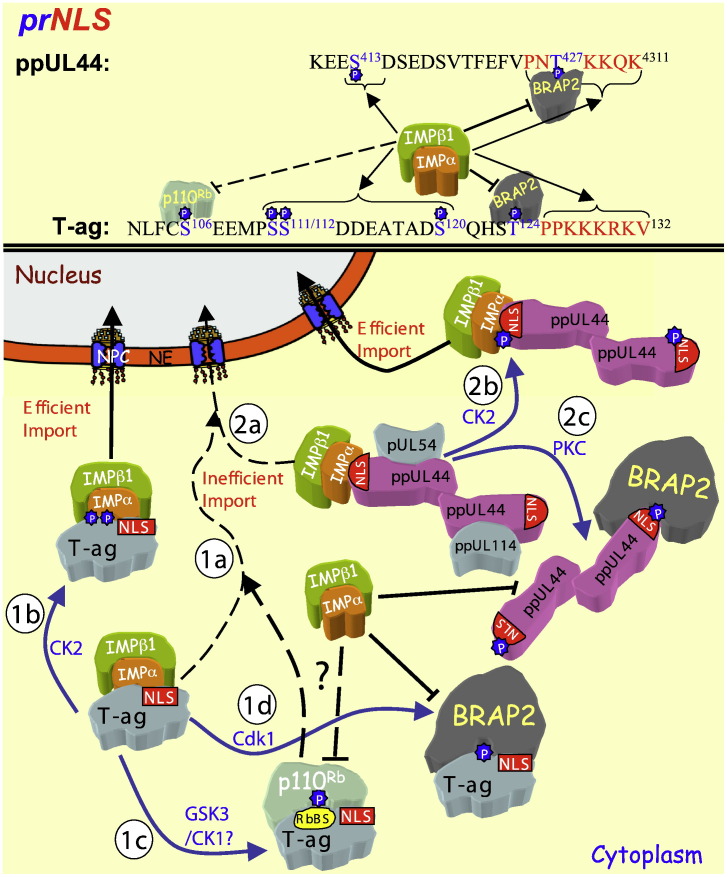
Fig. 4.
The regulation of viral protein nuclear import through phosphorylation near the NLS. The phosphorylation regulated NLSs (prNLSs) of SV40 T-ag and ppUL44 are shown (top — single letter amino acid code) with the regulatory phosphorylation sites (blue) and the NLS (red) highlighted, as well as the binding partners recognising them when phosphorylated (“P”). The T-ag NLS alone mediates IMPα/β1-mediated nuclear import, relatively inefficiently (black dotted arrow — 1a), but upon phosphorylation at serine111/112 by CK2 (blue arrow), IMPα/β1 is able to bind the T-ag NLS c. 50-fold better, to facilitate subsequent efficient nuclear import (1b). Phosphorylation at serine106 by GSK3/CK1 (blue arrow) allows p110Rb to bind T-ag at the RbBS, which leads to cytoplasmic retention and decreased nuclear import (1c). Phosphorylation at threonine124 by Cdk1 (blue arrow) allows BRAP2 to bind the T-ag NLS to prevent IMPα/β1 binding through intermolecular masking, and sequester T-ag in the cytoplasm (1d). (2a) HCMV ppUL44 IMPα/β1-mediated NLS-dependent nuclear import is inefficient (black dotted arrow) but upon phosphorylation at serine413 by CK2 (blue arrow), IMPα/β1 is able to bind the ppUL44 NLS with greater affinity to facilitate efficient nuclear import (2b). Phosphorylation at Thr427 by PK-C (blue arrow), enhances binding of ppUL44 to BRAP2 to prevent IMPα/β1 binding through intermolecular masking, and sequester ppUL44 in the cytoplasm and prevent nuclear import. Since ppUL44 may play a role in piggy-backing the HCMV proteins pUL54 and ppUL114 proteins into the nucleus early in infection, the various regulating mechanisms may apply to nuclear import of multiple HCMV proteins.