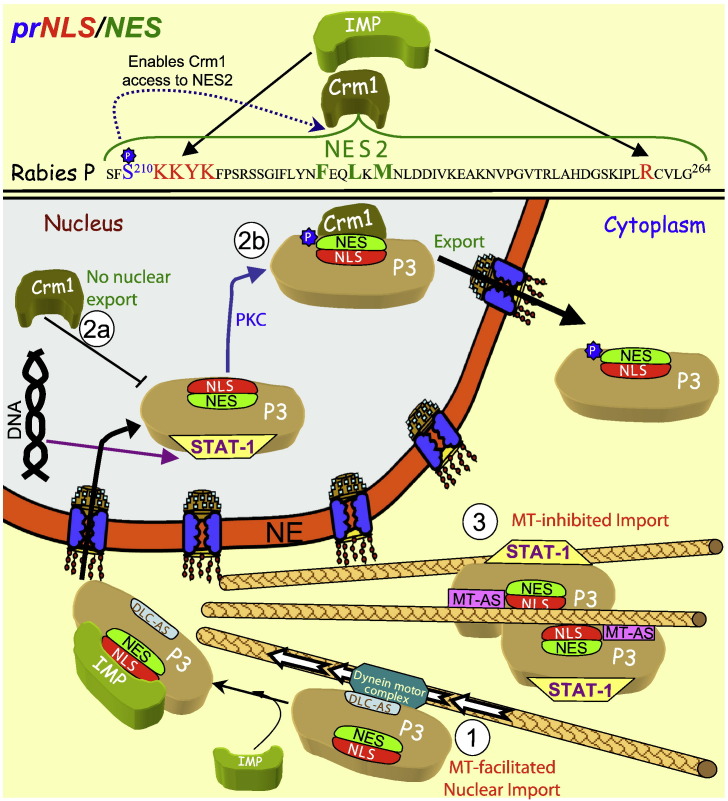
Fig. 6.
Regulation of nucleocytoplasmic shuttling of RV P3 protein to inhibit activity of the STAT-1 transcription factor in cytoplasmic and nuclear compartments. The prNLS/NES of RPP is shown (top), with the regulatory phosphorylation site (blue), NLS (red) and NES (green), highlighted, as well as the binding partners recognising them according to phosphorylation state (“P” indicating phosphorylation). In the absence of phosphorylation, P3 localises efficiently in the nucleus (1) through its NLS and the dynein light chain association sequence (DLC-AS), which confers binding to the microtubule (MT) motor dynein that acts to enhance IMP facilitated transport to the nucleus, where its role is to prevent DNA binding activity by the STAT-1 signalling molecule. P3 remains in the nucleus because NES2 is inaccessible (2a) until PKC phosphorylation of S210 induces conformation changes to render NES2 accessible to Crm1 (and mask the NLS) to permit Crm1 recognition and nuclear export (2b). Upon oligomerisation and binding to MTs through a MT-associating sequence (MT-AS) distinct from the DLC-AS, P3 remains cytoplasmic, acting to retain STAT-1 in the cytoplasm bound to MTs (3) and prevent its action in the anti-viral response.