Table 1.
Vancomycin and eremomycin type glycopeptides and their derivatives substituted at the X, Y and R positions 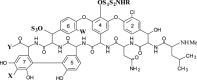
| LCTA | Code no. | X | Y | R | HIV-1 (CEM) |
FIPV (CRFK) |
SARS-CoV (Vero) |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| EC50 (μM) | IC50 (μM) | EC50 (μM) | CC50 (μM) | EC50 (μM) | CC50 (μM) | |||||
| Vancomycin (Van) and its derivatives W = Cl, S1 = Glc, S2 = vancosamine, S3 = H | ||||||||||
| 878 | 1 | H | OH | H | >250 | >500 | >100 | >100 | >100 | >100 |
| 854 | 2 | H | NHC10H21 | H | >10 | 30 ± 2 | >50 | 50 ± 1 | >80 | >80 |
| 892 | 3 | H | NH(CH2)3N+Me2C10H21 | H | 5.5 ± 0.7 | 172 ± 15 | >80 | >80 | 57 ± 14 | >80 |
| 893 | 4 | H | NHMe | H | >250 | >500 | >80 | >80 | >80 | >80 |
| 941 | 5 | CH2N[CH2CH2]2NBnBu-p | OH | H | 12 ± 3.5 | >100 | 30 ± 12 | >50 | 37 ± 2 | >100 |
| 1002 | 6 | H | OH | BnPhCI-p | NTa | NT | 20 | 61 | 22 ± 14 | >100 |
| Eremomycin (Ere) and its derivatives W = H, S1 = Glc, S2 = S3 = eremosamine | ||||||||||
| 516 | 7 | H | OH | H | >250 | >500 | >100 | >100 | >100 | >100 |
| 177 | 8 | H | CH3(CH2)2O | H | NT | NT | >80 | >80 | >80 | >80 |
| 200 | 9 | H | CH3(CH2)11O | H | NT | NT | >16 | 18 ± 4 | 27 ± 4 | >80 |
| 261 | 10 | H | NHMe | H | >250 | >500 | >80 | >80 | >80 | >80 |
| 284 | 11 | CH2NHC10H21 | OH | H | >20 | 24 ± 13 | >16 | 44 ± 3 | >40 | 54 ± 22 |
| 288 | 12 | CH2NMeCH2(CHOH)4CH2OH | OH | H | >250 | >250 | >80 | >80 | >80 | >80 |
| 289 | 13c | CH2NHC18H37 | OH | H | >10 | 15 ± 2 | 3.4 ± 1.4 | 15 ± 2 | 65 ± 48 | >80 |
| 302b | 14 | H | OH | H | NT | NT | >80 | >80 | >80 | >80 |
| 298 | 15c | CH2NHC12H25 | OH | H | >10 | 94 ± 4 | 8.9 ± 1 | 69 ± 8 | 31 ± 2 | >80 |
| 340 | 16 | H | NHC10H21 | H | >10 | 9.4 ± 4.7 | >16 | 19.5 ± 5 | >40 | 53 ± 15 |
| 353 | 17 | H | NHBnCI-p | H | >250 | >250 | >80 | >80 | >80 | >80 |
| 356 | 18 | CH2NHBnPh-p | OH | H | 17.5 ± 11 | >500 | >80 | >80 | 51 ± 17 | >100 |
| 368 | 19 | H | OH | C10H21 | >20 | 44 ± 2 | >16 | 51 ± 0 | 43 ± 26 | >80 |
| 375 | 20 | H | OH | BnCI-p | >250 | >250 | >80 | >80 | >80 | >80 |
| 512 | 21 | CH2N[CH2CH2]2NC10H21 | OH | H | >2 | 18 ± 11 | >10 | 16 ± 1 | >10 | 45 ± 8 |
| 518 | 22 | CH2N[CH2CH2]2NBnPh-p | OH | H | >10 | 53 ± 15 | >50 | 52 ± 6 | 14 ± 0 | >80 |
| 670 | 23 | H | NH(CH2)4CH(CONH(CH2)3NMe2)NHBnOBu-p | H | >50 | 117 ± 18 | >80 | >80 | >80 | >80 |
| 717 | 24 | H | OH | Bn(PhCI-p)-p | >10 | 44 ± 1 | >50 | 60 ± 5 | 33 ± 11 | >100 |
| 728 | 25 | CH2NH(CH2)3N+Me2C10H21 | NH(CH2)3NMe2 | H | 7.0 ± 0 | 27 ± 7 | >60 | 61 ± 0 | >40 | 45 ± 1 |
| 766 | 26 | CH2N[CH2CH2]2NBnBu-p | OH | H | 1.4 ± 0.6 | 96 ± 13 | 14 ± 4 | 58 ± 9 | 33 ± 1 | >80 |
| 768 | 27c | CH2N[CH2CH2]2NBnBu-p | NHMe | H | 0.43 ± .3 | 40 ± 4 | 5.4 ± 0.2 | 28 ± 3 | 14 ± 2 | 50 ± 6 |
| 770 | 28 | H | OC11H23 | H | >10 | 7.6 ± 1 | >16 | 35 ± 4 | 44 ± 9 | >80 |
| 784 | 29 | CH2NH(CH2)3N+Me2C10H21 | OH | H | >20 | 44 ± 3 | >80 | >80 | >80 | >80 |
| 826 | 30c | H | NH(CH2)3N+Me2 C10H21 | H | >20 | 38 ± 1 | 6.9 ± 0.4 | 48 ± 1 | >40 | 56 ± 21 |
| 827 | 31 | H | NH(CH2)6NH2 | H | >100 | >100 | >80 | >80 | >80 | >80 |
| 828 | 32 | H | NH(CH2)10NH2 | H | >20 | >100 | >80 | >80 | >80 | .80 |
| 829 | 33 | H | NHBnCH2NH2-p | H | >100 | >100 | >80 | >80 | >80 | >80 |
| 832 | 34 | H | NHBnNHC10H21-p | H | >4 | 5 ± 0.5 | >16 | 21 ± 8 | 22 ± 1 | >80 |
| 833 | 35 | H | NHBnN+Me2C10H21-p | H | >20 | 19 ± 8 | 14 ± 7 | 41 ± 5 | >40 | 46 ± 0 |
| 834 | 36 | H | NHCH2C5H4N+C10H21 | H | >20 | 35 ± 2 | >16 | 72 ± 9 | >80 | >80 |
| 837 | 37 | H | NHBnPhCI-p | H | >10 | 8.6 ± 0.2 | >20 | 27 ± 8 | 30 ± 24 | >80 |
| 846 | 38 | H | NHBnPh-p | H | >10 | 35 ± 1 | >50 | 53 ± 4 | 31 ± 8 | >100 |
| 847 | 39 | CH2NHBnPhCI-p | OH | H | 22.5 ± 3.5 | 106 ± 65 | 12 ± 3 | 54 ± 8 | 22 ± 12 | >100 |
| 848 | 40 | H | NHBnBu-p | H | >50 | 29 ± 12 | >50 | 52 ± 3 | 50 ± 3 | >100 |
| 864 | 41 | H | NHC7H15 | H | ≫50 | 182 ± 013 | >80 | >80 | 60 ± 19 | >100 |
| 869 | 42 | H | NHBnNBu2 | H | ≫10 | 30 ± 2 | 37 ± 16 | 49 ± 8 | 35 ± 19 | >100 |
| 921 | 43 | CH2N[CH2CH2]2NCOCH2NHBnBu-p | OH | H | >10 | 63 ± 29 | 42 | >80 | 45 ± 11 | >80 |
| 923 | 44 | H | N[CH2CH2]2NCH CH2 NHBnBu-p | H | >50 | >100 | >60 | 66 ± 12 | 31 ± 7 | >100 |
| 972 | 45 | H | NHCH((CH2)4NH2)CONHBnBu-p | H | >50 | 104 ± 11 | >16 | 73 ± 7 | >80 | >80 |
NT, not tested.
Carboxyeremomycin.
Antiviral values in italics denotes EC50 values equal or lower than 10 μg/ml. An asterix after the compound code no. indicates a selectivity (CC50/EC50) of >10 for the compound against either FIPV and/or SARS-CoV.
