Abstract
We have previously reported a new class of dendrimers with tryptophan (Trp) residues on the surface that show dual antiviral activities against HIV and enterovirus EV71. The prototype compound of this family is a derivative of pentaerythritol with 12 peripheral Trp groups and trivalent spacer arms. Here a novel series of dendrimers with divalent and tetravalent branched arms, instead of the trivalent ones present on the prototype, has been synthesized and its activity against HIV, EV71 and a panel of 16 different viruses and other pathogens has been determined. Convergent or divergent approaches have been used for the synthesis of these compounds. Our findings demonstrate that only compounds with tetravalent branched arms showed the same anti-HIV and anti-EV71 activity of the prototype (low micromolar) and even gain significant antiviral activity against new pathogens such as HSV-2, adenovirus-2, human corona virus and respiratory syncytial virus, being the first members of the Trp dendrimer family that showed activity against those viruses. As the prototype, these compounds also showed low-nanomolar activity against a representative EV71 clinical isolate. Experimental work carried on to determine the mode of action of the most potent IIa, containing tetravalent branched arms, demonstrated that it interacts with the viral envelopes of HIV, EV71 and HSV-2 and thus may prevent virus attachment to the host cell. These results support the interest of this new series of Trp dendrimers and qualify them as useful prototypes for the development of novel inhibitors of viral entry with broad antiviral spectrum.
Keywords: Antiviral agents, AIDS, HIV, EV71, HSV-2, Tryptophan
Graphical abstract
Highlights
-
•
Tryptophan (Trp) dendrimers with divalent and tetravalent branched arms have been synthesized.
-
•
Only dendrimers with tetravalent branched arms (IIa-IId) showed (sub)micromolar inhibitory activity against HIV and EV71.
-
•
IIa-IId inhibit a representative EV71 clinical isolate in the low-nanomolar range.
-
•
IIa-IId are the first members of the Trp dendrimer family that showed activity against new viruses such as HSV-2.
Human immunodeficiency virus (HIV) is the etiologic agent of the Acquired Immunodeficiency Syndrome (AIDS), a devastating infectious disease that continues to be a major global public health problem (UNAIDS DATA, 2018; WHO, 2018). The use of antiretroviral therapy (ART) has improved life expectancy and transformed HIV-1 infection into a manageable chronic disease (Arts, 2012; Broder, 2010; De Clercq, 2016; Mehellou, 2010; Volberding, 2010; Zhan, 2016). Despite this, long-term side effects, latent viral infection within reservoirs and the development of drug resistance, weaken the efficacy of all clinically available drugs (Drug Resistance Data Base, 2018; Margolis, 2014; Rojas, 2014) highlighting the fact that new inhibitors and antiviral treatment strategies are constantly needed (Badia, 2017; Barreé-Sinoussi, 2013; Gulick, 2019a; Kumari, 2013; Tintori, 2014). In this sense, the initial steps in the HIV replicative cycle (entry and/or fusion with the host cells) are very attractive targets (Esté, 2011; Kang, 2013; Klase, 2012; Lu, 2016; Micewicz, 2013; Wensing, 2012).
Currently there are only two FDA-approved entry inhibitors, Fuzeon (Ding, 2018; Fletcher, 2003), which targets the envelope glycoprotein gp41 and Maraviroc (Woollard, 2015), which targets the host cell receptor CCR5. However, despite the fact that the envelope glycoprotein gp120 is critical for HIV entry, there are still no approved drugs directed towards this target, being Fostemsavir, now in phase III clinical trials (Cahn, 2018; Gulick, 2018; 2019b; Meanwell, 2018; Vitoria, 2019) the most advanced drug in this class.
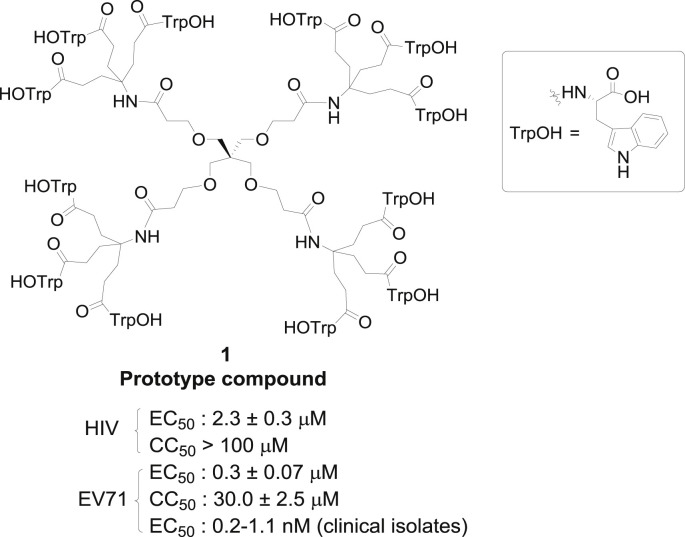
We have previously reported that dendrimer derivatives, with peripheral tryptophan (Trp) residues and trivalent aliphatic branched arms, exemplified by dendrimer 1 (Fig. 1 ), inhibit an early step in the replicative cycle of HIV by interacting with the gp120 and gp41 glycoproteins of the HIV envelope (Rivero-Buceta, 2015). Due to the current interest in HIV entry inhibitors associated with gp120 binding, our Trp dendrimers are attractive candidates for further development.
Fig. 1.
Structure of the dendrimer prototype 1.
Interestingly, dendrimer 1 also proved to be a potent, specific and selective inhibitor of the replication of the unrelated enterovirus A71 (EV-A71) (Rivero-Buceta, 2016), one of the major etiologic agents of hand, foot, and mouth disease (HFMD), which is occasionally associated with severe neurological complication in young children (Baggen, 2018; McMinn, 2002; Nadel, 2013; Solomon, 2010). So far, there are no antivirals approved to treat/prevent the HFMD infection.
Dendrimer 1 inhibits not only the laboratory strains of EV71 (BrCr strain) but also a large panel of clinical isolates (belonging to each of the genogroups) in the low-nanomolar/high-picomolar range (Rivero-Buceta, 2016). Recently, it was demonstrated that 1 targets residues of the structural protein VP1, particularly the region that forms the 5-fold vertex of the viral capsid, in turn blocking the binding of the virus to the host cell (Sun, 2019).
With respect to the SAR studies, modifications have been made in the central core and at the periphery of the prototype 1 (Rivero-Buceta, 2015, 2016; Martínez-Gualda, 2017). All of these modifications led to the conclusion that a multivalent presentation of Trps, with their respective indol side chain and free COOHs, is required for the antiviral activity against both HIV and EV71.
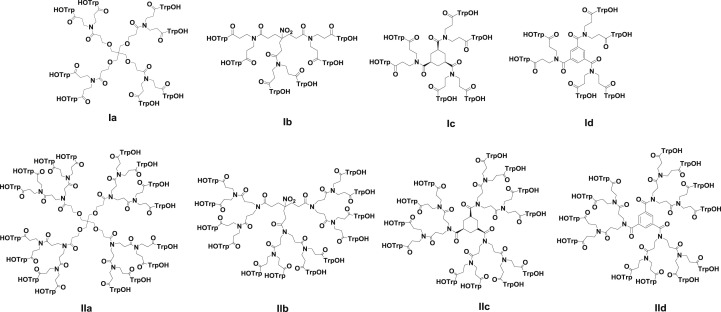
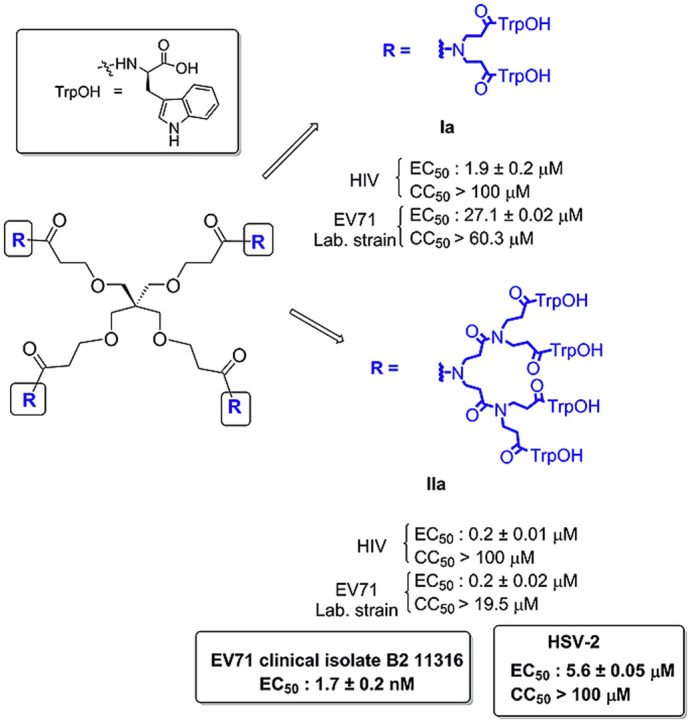
In all these compounds, trivalent spacer arms have been used to link the peripheral amino acids to the central core. To obtain more SAR information, new Trp dendrimers with lower and higher branching degree have now been prepared (Fig. 2 ). The lower branching degree, achieved through divalent branched arms (Fig. 2, compounds Ia-d), has been used to decrease peripheral congestion, and the highest branching degree, achieved through tetravalent branched arms (Fig. 2, compounds IIa-d), to maximize multivalent effects. Central scaffolds of different architecture and flexibility with four (Fig. 2, compounds Ia and IIa) or three (Fig. 2, compounds Ib-d and IIb-d) carboxylic acids as attachment points have been used.
Fig. 2.
Structure of dendrimers with divalent (Ia-d) and tetravalent (IIa-d) branched arms.
The dendrimers Ia-d and IIa-d were first evaluated for their inhibitory effects against the replication of HIV-1 and HIV-2 in CD4+ T cell cultures. Table 1 summarizes the results of this evaluation. The antiviral data of prototype 1 are included as reference, as well as dextran sulfate-5000 (DS-5000), a negatively charged HIV adsorption inhibitor (Baba, 1988) and pradimycin A (PRM-A), a gp120 carbohydrate-binding entry inhibitor (Balzarini, 2007).
Table 1.
Anti-HIV and anti-EV71 activity of the synthesized compounds.
| Compound | HIV-1 EC50a,c (μM) | HIV-2 EC50a,c (μM) | CC50b,c (μM) | EV71 EC50a,d (μM) | EV71 EC90e,d (μM) | CC50b,d (μM) |
|---|---|---|---|---|---|---|
| Ia | 1.9 ± 0.2 | 6.8 ± 5.8 | >100 | 27.1 | ND | >60 |
| Ib | 10 ± 6 | >100 | >100 | >82.2 | ND | >82 |
| Ic | 5.2 ± 2 | >100 | >100 | >85.1 | ND | >85 |
| Id | 3.6 ± 0.0 | >100 | >100 | 20.5 | ND | >57 |
| IIa | 0.2 ± 0.01 | 3.2 ± 0.10 | >100 | 0.20 ± 0.02 | 0.57 ± 0.03 | >19 |
| IIb | 1.1 ± 0.10 | 3.9 ± 0.30 | >100 | 1.44 ± 0.14 | 2.67 ± 0.05 | >26 |
| IIc | 1.0 ± 0.02 | 1.4 ± 0.02 | >100 | 1.51 ± 0.14 | 2.60 ± 0.19 | >27 |
| IId | 1.1 ± 0.30 | 1.3 ± 0.02 | >100 | 0.52 ± 0.03 | 0.95 ± 0.04 | >27 |
| 1 | 2.3 ± 0.30 | 6.6 ± 7.7 | >100 | 0.3 ± 0.10 | 0.5 ± 0.10 | >25 |
| DS-5000 | 0.07 ± 0.02 | 0.03 ± 0.01 | >20 | ND | ND | ND |
| PRM-A | 3.3 ± 1.2 | 5.9 ± 3.7 | >100 | ND | ND | ND |
| Pirodavir | ND | ND | ND | 0.3 ± 0.10 | 0.6 ± 0.2 | >100 |
Cell-based activity and toxicity assays were performed to determine EC50 and CC50 of selected compounds in the context of HIV and EV71 infection (see supplementary information for detailed protocols). Briefly, for EV71 RD cells were seeded in 96-well plate and infected at an MOI of 0.1. Decreasing concentration of compound were applied concomitantly with virus (1:3 dilution, concentration range 100 μM–0.1 μM). Cells were harvested at 3 days post infection and the virus-induced CPE inhibition was measured by MTS assay.
Data are the mean ± S.D. of 3 independient experiments in the two different susceptible cell types MT-4 and RD cells.
ND: Not Determined.
EC50: 50% Effective concentration, or the concentration required to inhibit virus-induced cytopathicity by 50%.
CC50: 50% Cytostatic concentration, or the concentration required to inhibit host cell viability by 50%.
In MT-4 cultures.
In RD cultures.
EC90: concentration of compound at which the EV71-induced cytopathic effect is reduced by 90%.
All the synthesized compounds showed micromolar activity against the replication of HIV-1, being the dendrimers IIa-d (EC50: 0.2–1.1 μM) and Ia (EC50: 1.9 μM), with 12, 16 or 8 peripheral Trp residues respectively, considerably more active than Ib-d (EC50: 3.6–10 μM), with only 6 peripheral Trp groups. In addition, IIa-d, as well as Ia, but not Ib-d, showed micromolar activity against HIV-2. Such differences in activity suggest that a minimum number of Trp residues is required for optimal HIV-1 and HIV-2 activity. No toxicity was observed for the host cells with any of these compounds at a concentration up to 100 μM.
Interestingly, dendrimer Ia (EC50: 1.9 μM), which contains 8 peripheral Trp residues and divalent spacer arms, is equally active against HIV-1 as prototype 1 (EC50: 2.3 μM), with 12 peripheral Trp residues and trivalent spacer arms. This result suggests that although the activity seems to be mainly related to the number of peripheral Trp residues, some other features of the molecules, such as the specific orientation of the Trp residues and/or the lower congestion of the peripheral amino acids, can be important for activity.
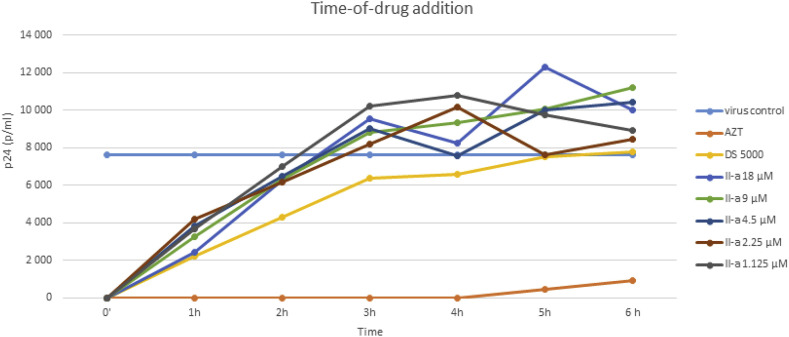
To determine at which stage of the HIV replication cycle the compounds act, a time-of-drug-addition study was performed with the most potent IIa. Different well-characterized anti HIV drugs were used as reference drugs. As inhibitor of the entry/fusion process the adsorption inhibitor DS5000 was used. AZT was used as reverse transcriptase inhibitor.
As it was shown in Fig. 3 full protective activity was achieved only when IIa was added early to the cells, almost within 1 h just before HIV was added. However, IIa lost its activity when it was added after HIV. This experiment clearly shows that IIa needs to be present in cell culture at the start of the virus infection (binding/adsorption period). Thus, we can conclude that compound IIa, like the prototype 1, interferes with early event(s) of the replicative cycle of HIV, very likely by inhibiting the entry of HIV into its susceptible human CD4+ T cells.
Fig. 3.
Time of drug-addition experiment in HIV-1 infected TZM-bl cells.
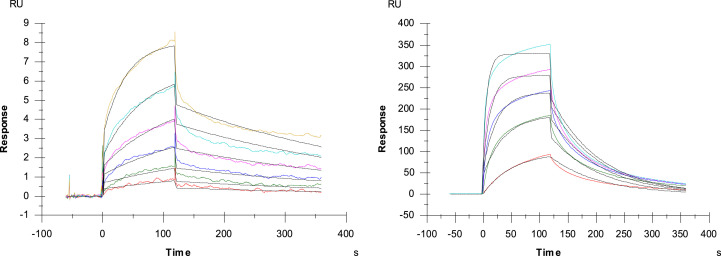
Next the potential binding of IIa to the virus-encoded gp120 envelope glycoprotein using surface plasmon resonance (SPR) technology was investigated. PRM-A was used as a positive control. As PRM-A, compound IIa clearly showed a high binding signal confirming the HIV interaction with gp120. Next, detailed SPR-directed affinity was determined using concentrations of IIa ranging between 3.1 and 0.1 μM. The apparent KD for the binding of IIa to gp120 (Fig. 4 and Table 2 ) was in the sub micromolar range (KD: 0.3 μM), being 23 times more pronounced than those found for the positive control, PRM-A (KD: 6.9 μM) and other members of the dendrimer family with trivalent branched arms (KD: 6.7 μM) (Rivero-Buceta, 2015).
Fig. 4.
Multiple cycle kinetics of IIa (left) and PRM-A (right). This figure shows one of the three replicate experiments. The latter was used as a positive control for the gp120 surface. The colored curves represent the real-time binding responses, the black curves indicate the calculated 1:1 Langmuir model fitting. The concentration of IIa ranged between 3.125 and 0.1 μM and that of PRM-A between 50 and 3.125 μM, both analytes were diluted using two-fold dilution steps. For both analytes, the ability of binding to gp120 was confirmed.
Table 2.
Apparent KD value for IIa-gp120 interaction.
| Compound | Ka (103) (1/M.s) | Ka (10−3) (1/s) | KD (10−6) (M) |
|---|---|---|---|
| IIa | 7.14 ± 2.41 | 2.26 ± 0.34 | 0.34 ± 0.86 |
| PRM-A | 2.76 ± 1.00 | 17.16 ± 4.21 | 6.92 ± 25.99 |
Average and standard deviation of kinetic parameters obtained from three replicate SPR experiments measuring the binding response between gp120 and IIa/PRM-A.
The new compounds Ia-d and IIa-d have also been evaluated to determine the selective antiviral activity (EC50) against the BrCr laboratory-adapted strain of EV71 in a virus-cell-based assay in rhabdomyosarcoma (RD) cells (Yamayoshi, 2009). Toxicity (CC50) was also assessed in a similar way with compound-treated, uninfected cells. Table 1 summarizes the results of this evaluation. The antiviral data of prototype 1 and the capsid binder pirodavir were included as references (Andries, 1992).
Dendrimers with divalent branched arms Ia-d were inactive (Ib and Ic) or much less active (Ia, Id) than the prototype 1, while dendrimers with tetravalent branched arms (IIa-d) showed a similar anti-EV71 activity (EC50: 1.5–0.2 μM) than prototype 1 (EC50: 0.3 μM). Among these, IIa (EC50: 0.2 μM) and IId (EC50: 0.5 μM) showed the highest anti-EV71 activity.
In this case, and in contrast to HIV, a wide difference in activity was observed between Ia (EC50: 27.1 μM), with branched divalent arms and 8 peripheral Trp residues, and prototype 1 (EC50: 0.3 μM), with trivalent branched arms and 12 peripheral Trp residues. This suggests that a greater degree of multivalency might be more important for EV71 than for HIV-1 activity.
In addition, the most active compounds, IIa and IId, were evaluated in a virus-cell-based assay against B2 11316, as a representative EV71 clinical isolate. Interestingly, and as previously reported for the Trp prototype 1 (EC50: 0.4 ± 0.0 nM) (Rivero-Buceta, 2016), the selected dendrimers IIa and IId are extremely potent inhibitors of this EV71 clinical isolate (activity in the nanomolar range) (EC50 for IIa: 1.7 ± 0.2 nM; EC50 for IId: 1.3 ± 0.06 nM) (Supporting Information, Table S2). These differences between the BrCr laboratory and the clinical strains were not observed when the cells were treated with a “classic” capsid binder like pirodavir (data not shown). This finding suggests that Trp dendrimers can act through a novel mechanism of action that is very different from that of “classic” capsid binders (Tijsma, 2014).
To determine if the novel Trp dendrimers IIa-d, whose structure is closely related to that of 1, would retain the same mechanism of action, cross resistance studies were performed with the most potent compound, IIa. This compound was evaluated for its inhibitory activity against the EV-71 variant (RES) that confers resistance to the prototype 1 (Sun, 2019) (Table 3 and Supporting Information, Fig. S6). Interestingly, IIa retained some antiviral activity (EC50: 2.4 μM) against this mutant virus strain, although this activity was at least 19-fold lower than against the wild-type virus. In contrast, rupintrivir, a 3C protease inhibitor, retained full antiviral activity against the mutant virus strain. Overall, these data suggest that IIa has a similar mechanism of antiviral activity (and resistance) as the parent prototype 1 and thus should interact with the five-fold axis of the capsid. However, it cannot be excluded that it may show additional interactions with amino acids at the five-fold axis, thus accounting for the residual activity against mutant EV71 viruses.
Table 3.
Susceptibility of reverse-engineered EV71 variant to prototype 1 and compound IIa.
| Compound | EV-A71 BrCr (WT) EC50a (μM) | VP1_S184T_ P246S (RES) EC50a (μM) | Fold resistance |
|---|---|---|---|
| 1 | 0.56 ± 0.06 | >14 | >25 |
| IIa | 0.12 ± 0.002 | 2.4 ± 0.6 | 19 |
| Rupintrivir | 0.014 ± 0.004 | 0.016 ± 0.006 | 1.1 |
Averages and standard deviation (SD) were calculated from data obtained from two independent antiviral assays.
All synthesized compounds Ia-d and IIa-d were also assayed against a variety of RNA and DNA viruses. Interestingly, dendrimers with tetravalent branched arms (IIa-d) showed significant activity against herpes simplex virus type 2 (HSV-2), adenovirus-2, human corona virus (HCoV-229 E) and respiratory syncytial virus. (Supporting Information, Table S1 and Figs. S1 to S4). In contrast, prototype 1 did not reveal any inhibitory activity against those viruses (data not shown) (Rivero-Buceta, 2016).
Compound IIb (EC50: 20 μM), which contains a very flexible central scaffold, showed less antiviral activity against HSV-2 than compounds IIa, IIc and IId (EC50: 5.6–8.9 μM), with more rigid central scaffolds. In contrast, IIb (EC50: 8.9 μM) was more active against HCoV-229 E than IIa, IIc and IId (EC50: 40–45 μM), showing that the structural requirements for activity are different between those viruses.
Particularly important is the activity showed by some of these compounds (II a, c, d) against herpes simplex virus type-2 (HSV-2) (genital herpes), a virus considered an important risk factor in HIV infection (Barnavas, 2012; Desai, 2015; Munawwar, 2016). Interestingly, an immunofluorescence experiment revealed that the most potent, IIa (Supporting Information, Table S1) inhibited the binding of a monoclonal antibody (mAb) directed to the HSV-2 envelope (Supporting Information, Table S3) supporting the interaction of IIa with the HSV-2 viral surface.
Finally, a broad anti-microbial screening was carried out for compounds Ia and IIa. The pathogens against which they were tested include Gram positive and negative bacteria and the yeast Candida albicans. None of the tested compounds showed inhibitory activity against these pathogens at sub-toxic concentrations.
In summary, a new series of Trp dendrimers containing divalent and tetravalent branched arms, instead of the trivalent ones present in the prototype 1, have been synthesized using convergent and divergent approaches. Our findings demonstrate that only compounds with tetravalent branched arms (IIa-d) showed a similar inhibitory activity against HIV and EV71 (laboratory strain) to that of the prototype 1 and interestingly, as previously reported for 1, are extremely potent inhibitors of the clinical EV71 isolate B211316 (low-nanomolar/high-picomolar potency). Notably, these compounds are also endowed with a significant inhibitory activity against HSV-2, HCoV-229 E and respiratory syncytial virus, being the first members of the Trp dendrimer family that showed activity against those viruses.
Experimental work carried on to determine the mode of action of the most potent IIa, demonstrated that it interacts with the viral envelopes of HIV, EV71 and HSV-2 probably preventing virus attachment to host cells. Maybe, the binding sites on the respective viral surfaces have adequate residues to establish electrostatic, hydrogen bond and van der Waals interactions with the negative charges and indole side-chains of the Trp residues.
All of these findings support the interest of this new family of Trp dendrimers and qualify them as attractive lead compounds to develop new viral entry inhibitors with broad antiviral spectrum.
Acknowledgements
This work has been supported by the Spanish Ministerio de Economía, Industria y Competitividad (MINECO) [project SAF2015-64629-C2-1-R (MINECO/FEDER)], the Spanish Agencia Estatal Consejo Superior de Investigaciones Científicas (CSIC, Project CSIC201680E079), “The Centers of Excellence” of the KU Leuven (EF-05/15 and PF-10/18), EU FP7 (FP7/2007–2013) Project EUVIRNA (Grant 408 Agreement 264286), EU FP7 SILVER (Contract HEALTH-F3-2010-260644), a grant from the Belgian Interuniversity Attraction Poles (IAP) Phase VII–P7/45 (BELVIR) and the EU FP7 Industry-Academia Partnerships and Pathways Project AIROPICO. The Spanish MEC/MINECO is also acknowledged for a grant to B.M.G and the China Scholarship Council (Grant 201403250056) for a grant to L.S. We also thank Charlotte Vanderheydt, Caroline Collard, Kim Donckers, Sandra Claes and Evelyne Van Kerckhove for help with the processing of the antiviral data and Arnaud Boonen and Sam Noppen with the generation of the SPR data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.antiviral.2019.06.006.
Appendix A. Supplementary data
Details regarding the synthetic procedures and characterization (1HNMR and 13CNMR) of the compounds and materials and methods (chemistry and biology) are included.
The following are the Supplementary data to this article:
References
- Andries K., Dewindt B., Snoeks J., Willebrords R., Van Eemeren K., Stokbroekx R., Janssen P.A.J. In vitro activity of pirodavir (R 77975), a substituted phenoxy pyridazinamine with broad-spectrum antipicornaviral activity. Antimicrob. Agents Chemother. 1992;36:100–107. doi: 10.1128/aac.36.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arts E.J., Hazuda D.J. HIV-1 antiretroviral drug therapy. Cold Spring Harbor Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a007161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba M., Pauwels R., Balzarini J., Arnout J., Desmyter J., De Clercq E. Mechanism of inhibitory effect of dextran sulfate and heparin on replication of human immunodeficiency virus in vitro. Proc. Natl. Acad. Sci. Unit. States Am. 1988;85:6132–6136. doi: 10.1073/pnas.85.16.6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badia R., Ballana E., Esté J.A., Riveira-Muñoz E. Antiviral treatment strategies based on gene silencing and genome editing. Curr. Opin. Virol. 2017;24:46–54. doi: 10.1016/j.coviro.2017.04.001. [DOI] [PubMed] [Google Scholar]
- Baggen J., Thibaut H.J., Strating J.R.P.M., van Kuppeveld F.J.M. The life cycle of non-polio enteroviruses and how to target it. Nat. Rev. Microbiol. 2018;16:368–381. doi: 10.1038/s41579-018-0005-4. [DOI] [PubMed] [Google Scholar]
- Balzarini J., Laethem K.V., Daelemans D., Hatse S., Bugatti A., Rusnati M., Igarashi Y., Oki T., Schols D. Pradimicin A, a carbohydrate-binding nonpeptidic lead compound for treatment of infections with viruses with highly glycosylated envelopes, such as human Immunodeficiency virus. J. Virol. 2007;81:362–373. doi: 10.1128/JVI.01404-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnavas R.V., Celum C. Infectious co-factors in HIV-1 transmission Herpes Simplex Virus type-2 and HIV-1: new insights and interventions. Curr. HIV Res. 2012;10:228–237. doi: 10.2174/157016212800618156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barré-Sinoussi F., Ross A.L., Delfraissy J.-F. Past, present and future: 30 years of HIV research. Nat. Rev. Microbiol. 2013;12:877–883. doi: 10.1038/nrmicro3132. [DOI] [PubMed] [Google Scholar]
- Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antivir. Res. 2010;85:1–18. doi: 10.1016/j.antiviral.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahn P., Fink V., Patterson P. Fostemsavir: a new CD4 attachment inhibitor. Curr. Opin. HIV AIDS. 2018;13:341–345. doi: 10.1097/COH.0000000000000469. [DOI] [PubMed] [Google Scholar]
- De Clercq E., Li G. Approved antiviral drugs over the past 50 years. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai D.V., Kulkami S.S. Herpex simplex virus: the interplay between HSV, host and HIV-1. Viral Immunol. 2015;28:546–555. doi: 10.1089/vim.2015.0012. [DOI] [PubMed] [Google Scholar]
- Ding X., Zhu Y., Chong H., Cui S., Zhang X., He J., Wang X., He Y. Structural and functional characterization of HIV-1 cell fusion inhibitor T20. AIDS. 2018;33:1–11. doi: 10.1097/QAD.0000000000001979. [DOI] [PubMed] [Google Scholar]
- Drug resistance database 2018. https://hivdb.stanford.edu/dr-summary/resistance-notes/NNRTI/
- Esté J.A. Inhibition of HIV entry. In: De Clercq E., editor. vol. 50. Wiley-VCH Verlag GmbH&Co. KGaA; Weinheim, Germany: 2011. pp. 29–50. (Antiviral Drug Strategies, Methods and Principles in Medicinal Chemistry). [Google Scholar]
- Fletcher C.V. Enfuvirtide, a new drug for HIV infection. Lancet. 2003;361:1577–1578. doi: 10.1016/S0140-6736(03)13323-5. [DOI] [PubMed] [Google Scholar]
- Gulick R.M. New HIV drugs: 2018 and beyond. Curr. Opin. HIV AIDS. 2018;13:291–293. doi: 10.1097/COH.0000000000000478. [DOI] [PubMed] [Google Scholar]
- Gulick R.M. Investigational antiretroviral drugs: what is coming down the pipeline. Top. Antivir. Med. 2019;25:127–132. [PMC free article] [PubMed] [Google Scholar]
- Gulick R.M., Flexner Ch. Long-acting HIV drugs for treatment and prevention. Annu. Rev. Med. 2019;70:137–150. doi: 10.1146/annurev-med-041217-013717. [DOI] [PubMed] [Google Scholar]
- Kang Y., Guo J., Chen Z. Closing the door to human immunodeficiency virus. Protein Cell. 2013;4:86–102. doi: 10.1007/s13238-012-2111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klase J. The molecular basis of HIV entry. Cell Microbiol. 2012;14:1183–1192. doi: 10.1111/j.1462-5822.2012.01812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari G., Singh R.K. Anti-HIV drug development: structural features and limitations of present day drugs and future challenges in the successful HIV/AIDS treatment. Curr. Pharmaceut. Des. 2013;19:1767–1783. doi: 10.2174/13816128113199990295. [DOI] [PubMed] [Google Scholar]
- Lu L., Fei Y., Lifeng C., Asim K.D., Shibo J. Development of small-molecule HIV entry inhibitors specifically targeting gp120 or gp41. Curr. To. Med. Chem. 2016;16:1074–1090. doi: 10.2174/1568026615666150901114527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis A.M., Heverling H., Pham P.A., Stolbach A. A review of the toxicity of HIV medications. J. Med. Toxicol. 2014;10:26–39. doi: 10.1007/s13181-013-0325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Gualda B., Sun L., Rivero-Buceta E., Flores A., Quesada E., Balzarini J., Noppen S., Liekens S., Schols D., Neyts J., Leyssen P., Mirabelli C., Camarasa M.-J., San-Félix A. Structure-activity relationship studies on a Trp dendrimer with dual activities against HIV and enterovirus A71. Modifications on the amino acid. Antivir. Res. 2017;139:32–40. doi: 10.1016/j.antiviral.2016.12.010. [DOI] [PubMed] [Google Scholar]
- McMinn P.C. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol. Rev. 2002;26:91–107. doi: 10.1111/j.1574-6976.2002.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Meanwell N.A., Krystal M.R., Nowicka-Sans B., Langley D.R., Conlon D.A., Eastgate M.D., Grasela D.M., Timmins P., Wang T., Kadow J.F. Inhibitors of HIV-1 attachment: the discovery and development of Temsavir and its prodrug Fostemsavir. J. Med. Chem. 2018;61:62–80. doi: 10.1021/acs.jmedchem.7b01337. [DOI] [PubMed] [Google Scholar]
- Mehellou Y., De Clercq E. Twenty-six years of anti-HIV drug discovery: where do we stand and where do we go? J. Med. Chem. 2010;53:521–538. doi: 10.1021/jm900492g. [DOI] [PubMed] [Google Scholar]
- Micewicz E.D., Ruchala P. Inhibitors of HIV-1 entry. Curr. Pharmaceut. Des. 2013;10:1784–1799. doi: 10.2174/1381612811319100003. [DOI] [PubMed] [Google Scholar]
- Munawwar A., Singh S. Human herpesviruses as copathogens of HIV infection, their role in HIV transmission, and disease progression. J. Lab. Phys. 2016;8:5–18. doi: 10.4103/0974-2727.176228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel S. Hand, foot, mouth, brainstem and heart disease resulting from enterovirus 71. Crit. Care Med. 2013;41:1821–1822. doi: 10.1097/CCM.0b013e318291cb2d. [DOI] [PubMed] [Google Scholar]
- Rivero-Buceta E., Doyagüez E.G., Colomer I., Quesada E., Mathys L., Noppen S., Liekens S., Camarasa M.-J., Pérez-Pérez M.-J., Balzarini J., San-Félix A. Tryptophan dendrimers that inhibit HIV replication, prevent virus entry and bind to the HIV envelope glycoproteins gp120 and gp41. Eur. J. Med. Chem. 2015;106:34–43. doi: 10.1016/j.ejmech.2015.10.031. [DOI] [PubMed] [Google Scholar]
- Rivero-Buceta E., Sun L., Martínez-Gualda B., Doyagüez E.G., Donkers K., Quesada E., Camarasa M.-J., Delang L., San-Félix A., Neyts J., Leyssen P. Optimization of a class of tryptophan dendrimers that inhibit HIV replication leads to a selective, specific and low-nanomolar inhibitor of clinical isolates of enterovirus A71. Antimicrob. Agents Chemother. 2016;60:5064–5067. doi: 10.1128/AAC.00626-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas P., Holguín A. Drug resistance in the HIV-1-infected paediatric population worldwide: a systematic review. J. Antimicrob. Chemother. 2014;69:2032–2042. doi: 10.1093/jac/dku104. [DOI] [PubMed] [Google Scholar]
- Solomon T., Lewthwaite P., Perera D., Cardosa M.J., McMinn P., Ooi M.H. Virology, epidemiology, pathogenesis and control of enterovirus 71. Lancet Infect. Dis. 2010;10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- Sun L., Lee H., Thibaut H.J., Rivero-Buceta E., Bator C., Martinez-Gualda B., Dallmeiera K., Delang L., Leyssen P., Gago F., San-Félix A., Hafenstein S., Mirabelli C., Neyts J. Viral engagement with host (co-)receptors blocked by a novel class of tryptophan dendrimers that targets the 5-fold-axis of the enterovirus-A71 capsid. PLoS Pathog. 2019;15(5) doi: 10.1371/journal.ppat.1007760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tijsma A., Franco D., Tucker S., Hilgenfeld R., Froeyen M., Leyssen P., Neyts J. The capsid binder vapendavir and the novel protease inhibitor SG85 inhibit enterovirus 71 replication. Antimicrob. Agents Chemother. 2014;58:6990–6992. doi: 10.1128/AAC.03328-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintori C., Brai A., Fallacara A.L., Fazi R., Schenone S., Botta M. Protein–protein interactions and human cellular cofactors as new targets for HIV therapy. Curr. Opin. Pharmacol. 2014;18:1–8. doi: 10.1016/j.coph.2014.06.005. [DOI] [PubMed] [Google Scholar]
- UNAIDS data 2018. http://www.unaids.org/en/topic/data
- Vitoria M., Rangaraj A., Ford N., Doherty M. Current and future priorities for the development of optimal HIV drugs. Curr. Opin. HIV AIDS. 2019;14:143–149. doi: 10.1097/COH.0000000000000527. [DOI] [PubMed] [Google Scholar]
- Volberding S., Deeks G. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376(9734):49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- Wilen C.B., Tilton J.C., Doms R.W. Molecular mechanisms of HIV entry. Adv. Exp. Med. Biol. 2012;726:223–242. doi: 10.1007/978-1-4614-0980-9_10. [DOI] [PubMed] [Google Scholar]
- WHO . 2018. World Health Organization HIV/AIDS Fact Sheet [Internet]https://www.who.int/en/news-room/fact-sheets/detail/hiv-aids [Google Scholar]
- Woollard S.M., Kanmogne G.D. Maraviroc: a review of its use in HIV infection and beyond. Drug Des. Dev. Ther. 2015;9:5447–5468. doi: 10.2147/DDDT.S90580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamayoshi S., Yamashita Y., Li J., Hanagata N., Minowa T., Takemura T., Koike S. Scavenger receptor B2 is a celular receptor for enterovirus 71. Nat. Med. 2009;15:798–801. doi: 10.1038/nm.1992. [DOI] [PubMed] [Google Scholar]
- Zhan P., Pannecouque C., De Clercq E., Liu X. Anti-HIV drug discovery and development: current innovations and future trends. J. Med. Chem. 2016;59:2849–2878. doi: 10.1021/acs.jmedchem.5b00497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.