Short abstract
Objectives
Glomerulonephritis is a serious kidney disease that can induce end-stage renal failure. The aberrant proliferation of mesangial cells is a cause of glomerulonephritis. Traditional Chinese medicines, such as Astragalus and Salvia miltrorrhiza, play important roles in the treatment of kidney-related diseases. However, the effects of a combination of Astragalus and S. miltrorrhiza-containing traditional Chinese medicines (Polygala fallax Hemsl and compound Sanqi granules) on glomerulonephritis are unclear.
Methods
HRM cells (human mesangial cells) were stimulated with lipopolysaccharide to simulate glomerulonephritis. Separately, compound Sanqi granules and P. fallax Hemsl were administered to nude mice in various combinations. Serum was collected from the treated mice and added to HRM cells; the proliferation and apoptosis characteristics of the cells were assessed.
Results
The proliferation of HRM cells was inhibited after exposure to serum from treated mice. Exposure to serum from treated mice moderately induced apoptosis of HRM cells and lowered the expression levels of TNF-α, IL-1β, and IL-6.
Conclusions
Combination treatment with compound Sanqi granules and P. fallax Hemsl exerts a therapeutic effect on glomerulonephritis by inhibiting the proliferation of mesangial cells, while inducing apoptosis in those cells.
Keywords: Glomerulonephritis, Polygala fallax Hemsl, compound Sanqi granules, glomerular mesangial cells, proliferation, apoptosis
Introduction
Nephritis is a disease that can lead to renal failure.1 A form of nephritis, glomerulonephritis, is induced by mesangial hyperplasia. Glomerular mesangial cells play critical roles in normal kidney function. However, uncontrolled proliferation of glomerular mesangial cells can lead to excessive production of extracellular matrix, thereby promoting the occurrence and development of glomerulonephritis.2 This abnormal proliferation of mesangial cells occurs in various glomerular diseases.3 Therefore, inhibiting the abnormal proliferation of glomerular mesangial cells is critical for delaying the progression of renal disease. Many studies have shown that glomerulonephritis is regulated by immune and inflammatory responses.4 Thus, some inflammatory factors are involved in the occurrence of glomerulonephritis and contribute to glomerular damage.5,6
Some drugs can alleviate the damage caused by glomerulonephritis, but most have strong toxicity and side effects.7,8 Consequently, a new pharmacotherapy is needed for treatment of glomerulonephritis. Compound Sanqi granules are a compound Chinese medicine consisting of pseudo-ginseng, Astragalus, and Salvia. Astragalus has been shown to exhibit a therapeutic effect on nephritis9 and Salvia miltiorrhiza has been shown to protect against lupus nephritis when combined with other drugs.10 The active ingredient in Polygala fallax Hemsl, another type of Chinese traditional medicine, was proven to inhibit the proliferation of vascular smooth muscle cells in vitro.11 However, it is unknown whether P. fallax Hemsl can suppress the proliferation of mesangial cells, thereby inhibiting the occurrence and development of glomerulonephritis. Additionally, prior studies have not shown whether the combination of compound Sanqi granules and P. fallax Hemsl could enhance the curative effects of these compound drugs.
Lipopolysaccharide (LPS), a component of Gram-negative bacteria, is commonly used to induce abnormal proliferation of glomerular mesangial cells.12 Because there are many impurities in crude extracts used in traditional Chinese medicine, a serum pharmacology method13 is used in which medicines are administered to animals; the serum is then isolated and used for assessment of glomerular mesangial cell activity in vitro. This method is presumed to avoid interference from the impurities in crude extracts and directly assess the pharmacological effects of the tested medicines. In this study, we used the serum pharmacology method to investigate the effects of P. fallax Hemsl and compound Sanqi granules on glomerulonephritis in vitro, specifically to determine whether the combination of these drugs could inhibit the proliferation of glomerular mesangial cells.
Materials and methods
Production of medicine serum
This research protocol was approved by the ethics committee of Guilin Second People’s Hospital. Thirty healthy male nude mice were obtained from Shanghai Lingchang Biotechnology Company (Shanghai, China). All mice were housed under specific pathogen-free conditions and cared for in accordance with the animal experimental guidelines designated by the National Institutes of Health. Mice were divided into six equal groups, which received the following compound medicines: pseudo-ginseng 20 g, S. miltiorrhiza 20 g, and Astragalus 60 g (group 1); P. fallax Hemsl 50 g and Astragalus 50 g (group 2); P. fallax Hemsl 60 g and pseudo-ginseng 20 g (group 3); P. fallax Hemsl 60 g and S. miltiorrhiza 20 g (group 4); pseudo-ginseng 20 g, S. miltiorrhiza 20 g, Astragalus 60 g, and P. fallax Hemsl 60 g (group 5); and P. fallax Hemsl 50 g (group 6). These drugs were all in powdered form (provided by Guangdong Yifang Pharmaceutical Co. Ltd., Foshan, China) and were dissolved in distilled water at final concentrations sufficient to ensure that the mice in each group received the weights of medicine listed above with a 10-mL/kg dose volume. To facilitate the absorption of the drugs, the mice were fasted for 12 hours before administration. Drugs were administered by the intragastric route for 8 consecutive days (10 mL/kg, once per day). At 2 hours after the last dose of the drugs, mice were anesthetized with chloroform (Chenniao Company, Shanghai, China) and blood was collected via intracardiac puncture. The blood was placed at room temperature for 1 hour and centrifuged at 12,500 × g for 10 minutes after coagulation, then heated at 56°C for 30 minutes to inactivate the complement. Subsequently, the serum fraction of the blood was sterile filtered using a 0.22-µm microporous membrane and stored at −80°C.
Cell culture and treatment
Human mesangial cells (HRM cells) were obtained from the Shanghai Academy of Sciences (Shanghai, China). The cells were cultured in RPMI 1640 medium (Hyclone Laboratories Inc., Logan, UT, USA) supplemented with 10% FBS (Gibco Cell Culture, Carlsbad, CA, USA) and incubated at 37°C with 5% CO2. LPS (Sigma-Aldrich, St. Louis, MO, USA) and serum from treated mice were added to the cell culture medium during the experiment.
MTT assays
HRM cells were seeded into 96-well plates (Corning, Corning, NY, USA) at a density of 2 × 103 cells per well. After 24 hours, cells were simultaneously treated with LPS (1 µg/mL) and serum from treated mice. Control cells were only treated with serum from treated mice. The cells were cultured for 24, 48, 72, 96, and 120 hours. They were then incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (Thermo Fisher Scientific, Inc., Rockford, IL, USA) for 4 hours and the absorbance was measured at 490 nm.
Apoptosis assays
HRM cells were seeded in a culture dish and grown to approximately 70% confluence. They were then stimulated simultaneously with the LPS and serum from the treated mice. Subsequently, cells were washed with 1× binding buffer (eBioscience, San Diego, CA, USA), then incubated with Annexin V-APC and propidium iodide (eBioscience). The apoptosis ratios of the stimulated cells were detected by flow cytometry (Guava EasyCyte; Millipore, Billerica, MA, USA).
Western blotting
Cells were grown and stimulated as described for the apoptosis assays. Total protein was extracted using RIPA lysate (Beyotime Biotech, Inc., Jiangsu, China). The bicinchoninic acid method (Beyotime Biotech, Inc.) was used to determine protein concentrations in the cell lysates. Protein samples (40 µg) were denatured by heating at 99°C for 10 minutes, then separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Beyotime Biotech, Inc.). Separated proteins were transferred to a polyvinylidene difluoride membrane and the membrane was blocked with 5% skim milk powder. The membrane was incubated with one of the following primary antibodies at 4°C overnight: anti-TNF-α (Cat. No. ab6671), anti-IL-1β (Cat. No. ab2105), anti-IL-6 (Cat. No. ab6672), anti-Bax (Cat. No. ab32503), anti-Bcl-2 (Cat. No. ab692), anti-caspase-3 (Cat. No. ab13847), or anti-GAPDH (Cat. No. ab8245) (all primary antibodies were from Abcam [Cambridge, MA, USA]). The membranes were washed three times with phosphate-buffered saline with Tween, then incubated with secondary antibody (rabbit IgG [Cat. No. 7074S] and mouse IgG [Cat. No. 7076S]; both secondary antibodies were from Cell Signaling Technology, Danvers, MA, USA) at room temperature for 2 hours. The membranes were again washed with phosphate-buffered saline with Tween. Pierce™ ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc.) was used to develop the antibody signal on the membranes.
Quantitative PCR
Cells were grown and stimulated as described for the apoptosis assays. Total RNA was extracted with Trizol (Thermo Fisher Scientific, Inc.), then reverse-transcribed into cDNA using a reverse transcription kit (Takara Bio, Inc., Shiga, Japan). Quantitative PCR was performed with the 7500 Real-time PCR System (Applied Biosystems, Foster City, CA, USA). The relative levels of target genes were calculated using the 2−ΔΔCT method. The following primers were used in this experiment: TNF-α Forward, AACATCCAACCTTCCCAAACGC; TNF-α Reverse, TGGTCTCCAGATTCCAGATGTCAGG; IL-6 Forward, CGCCTTCGGTCCAGTTGCC; IL-6 Reverse, GCCAGTGCCTCTTTGCTGCTTT; β-actin Forward, GACTTAGTTGCGTTACACCCTTTCTTG; β-actin Reverse, CTGTCACCTTCACCGTTCCAGTTTT.
Statistical analysis
All statistical analyses were performed with GraphPad Prism, version 7.0 (GraphPad Software, Inc., La Jolla, CA, USA). Comparisons were made using Student’s t-test. Differences with P<0.05 were considered statistically significant. Data are presented as mean ± standard deviation and all experiments were performed three times.
Results
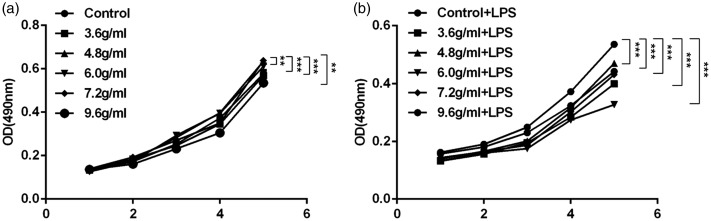
Serum from mice treated with a combination of compound Sanqi granules and Polygala fallax Hemsl inhibited aberrant proliferation of glomerular mesangial cells
MTT assays were performed to detect the proliferation of cells exposed to serum from mice treated with different combinations of the test drugs. As shown in Figure 1a, serum from mice treated with the combination of P. fallax Hemsl and compound Sanqi granules more strongly suppressed the proliferation of HRM cells, compared with serum from mice treated with P. fallax Hemsl alone (P < 0.01 for all). After the cells were stimulated with LPS, the difference in inhibitory efficacy was enhanced between the control and treatment groups (P < 0.001 for all) (Figure 1b).
Figure 1.
Proliferation of HRM cells was inhibited by exposure to serum from treated mice. (a) Proliferation of HRM cells was suppressed by exposure to serum from treated mice (n=3, ± standard deviation). (b) Growth rate of LPS-stimulated HRM cells was slowed by exposure to serum from treated mice (n=3, ± standard deviation). **P<0.01, ***P<0.001. Abbreviations: LPS, lipopolysaccharide; OD, optical density.
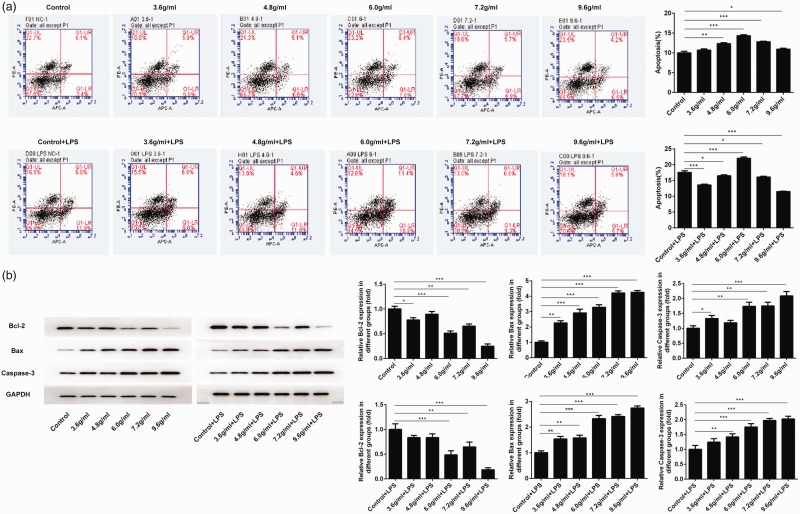
Serum from mice treated with a combination of compound Sanqi granules and Polygala fallax Hemsl moderately induced apoptosis of glomerular mesangial cells
Because some studies have shown that the apoptosis of glomerular mesangial cells is a critical mechanism by which the body prevents excessive proliferation of these cells,14,15 we examined the effect of serum from treated mice on the apoptosis of glomerular mesangial cells in vitro. The ratio of apoptotic cells was measured after exposure to serum from treated mice. Notably, serum from mice treated with P. fallax Hemsl (Control group) caused less induction of apoptosis, compared with serum from mice that had received combination treatment (P < 0.05 for all) (Figure 2a). However, after stimulation with LPS, the cells exposed to serum from mice treated with 6.0 g/mL of the drugs had a higher apoptosis ratio, compared with the control group (P < 0.001) (Figure 2a); the other cells had lower apoptosis ratios, compared with the control group (P < 0.05 for all). Expression levels of apoptosis-related proteins were then assessed by western blotting. As shown in Figure 2b, the protein expression levels of Bax and caspase-3 were increased after exposure to serum from treated mice (P < 0.001 and P < 0.05, respectively, for all), while the levels of Bcl-2 were reduced (P < 0.05 for all). After stimulation with LPS, similar protein expression tendencies were observed.
Figure 2.
The apoptosis rate of HRM cells was enhanced after exposure to serum from treated mice. (a) Exposure to serum from treated mice induced apoptosis in HRM cells (n=3, ± standard deviation). (b) Levels of apoptosis-related proteins were promoted after exposure to serum from treated mice (n=3, ± standard deviation). *P<0.05, **P<0.01, ***P<0.001. Abbreviation: LPS, lipopolysaccharide.
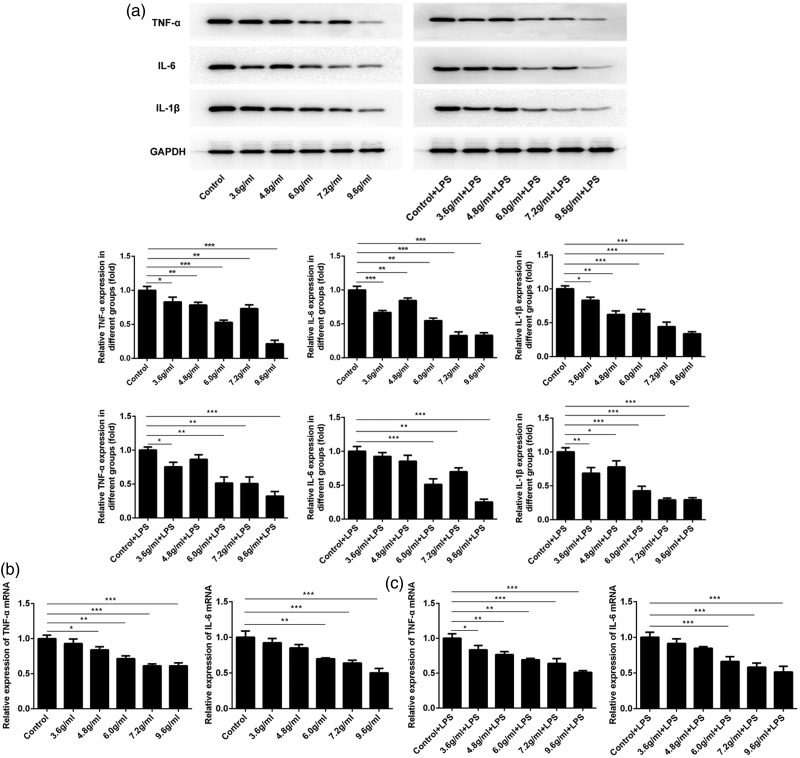
Expression levels of TNF-α, IL-6, and IL-1β were reduced in cells exposed to serum from mice treated with a combination of compound Sanqi granules and Polygala fallax Hemsl
Because inflammation is often accompanied by changes in the levels of many inflammatory factors, the expression of inflammation-related indicators was examined in HRM cells. As shown in Figure 3a, the protein expression level of TNF-α was lower in the cells exposed to serum from mice treated with the combination of P. fallax Hemsl and compound Sanqi granules than in cells exposed to serum from mice treated with P. fallax Hemsl alone (P < 0.05 for all). The protein expression levels of IL-6 and IL-1β showed similar tendencies (P < 0.01 and P < 0.05, respectively, for all). Moreover, the expression levels of TNF-α, IL-6, and IL-1β decreased in LPS-stimulated HRM cells after exposure to serum from treated mice (P < 0.05, P < 0.01, and P < 0.05, respectively, for all). In addition, the mRNA expression levels of TNF-α and IL-6 were determined by quantitative PCR. Figure 3b and c show that exposure to serum from treated mice caused reductions in the mRNA expression levels of TNF-α and IL-6 in HRM cells (P < 0.05 and P < 0.01, respectively, for all). Following stimulation with LPS, the mRNA expression levels of TNF-α and IL-6 were also inhibited in the cells exposed to serum from treated mice (P < 0.05 and P < 0.001, respectively, for all).
Figure 3.
Expression levels of TNF-α, IL-6, and IL-1β were reduced after exposure to serum from treated mice. (a) Immunoblotting results for TNF-α, IL-6, IL-1β, and GAPDH are shown. (b) mRNA levels of TNF-α and IL-6 declined after exposure to serum from treated mice (n=3, ± standard deviation). *P<0.05, **P<0.01, ***P<0.001. Abbreviation: LPS, lipopolysaccharide.
Discussion
Many factors contribute to the onset of nephritis; in particular, glomerulonephritis is caused by the abnormal proliferation of glomerular mesangial cells. Previous research demonstrated that immune disorders can cause nephritis.4,16 In recent years, many Chinese medicines have been shown to exhibit therapeutic effects on nephritis and nephropathy; these medicines include Astragalus, S. miltiorrhiza, and ginsenoside Rg1.9,10,17,18 Compound Sanqi granules consist of pseudo-ginseng, Astragalus, and S. miltiorrhiza. P. fallax Hemsl is often used in treatment of hypercholesterolemia and inflammation.19 Our study investigated whether the combination of compound Sanqi granules and P. fallax Hemsl was effective for treatment of glomerulonephritis.
Glomerular mesangial cells are intrinsic cells in the kidney. Under normal physiological conditions, glomerular mesangial cells proliferate in a slow manner; aberrant proliferation under pathological conditions eventually causes damage to renal function.20–22 In this study, we found that the proliferation of glomerular mesangial cells was suppressed by exposure to serum from treated mice, regardless of additional stimulation with LPS; this indicated that combined treatment with compound Sanqi granules and P. fallax Hemsl inhibited the proliferation of glomerular mesangial cells. Our findings suggest that these drugs may be effective for treatment of glomerulonephritis. The induction of apoptosis has been identified as another method to suppress the proliferation of glomerular mesangial cells.14,15 In our study, we found that exposure to serum from treated mice induced apoptosis of glomerular mesangial cells that had been stimulated with LPS, thereby inhibiting the abnormal proliferation of these cells. This may be useful for treatment of various kidney diseases caused by abnormal proliferation of glomerular mesangial cells.
TNF-α, IL-6, and IL-1β are inflammatory cytokines that are upregulated in the context of inflammation,23–26 such as glomerulonephritis. Reinioside C, a plant extract of P. fallax Hemsl, has been reported to inhibit TNF-α expression, thereby protecting against inflammatory injury to the vascular endothelium.27 Dihydrotanshinone I, a natural product isolated from Salvia, was also demonstrated to alleviate pulmonary inflammation induced by crystalline silica.28 In the present study, we investigated the levels of TNF-α, IL-6, and IL-1β. We found that expression levels of TNF-α, IL-6, and IL-1β were gradually reduced in glomerular mesangial cells after exposure to serum from treated mice, regardless of stimulation with LPS. Therefore, combined treatment with compound Sanqi granules and P. fallax Hemsl may limit the development of glomerulonephritis.
Overall, our results indicated that combined treatment with compound Sanqi granules and P. fallax Hemsl suppressed the proliferation of glomerular mesangial cells and partly induced apoptosis in these cells. Analysis of the expression levels of TNF-α, IL-6, and IL-1β illustrated that treatment with compound Sanqi granules and P. fallax Hemsl suppressed inflammatory cytokine expression in glomerular mesangial cells. Therefore, this treatment could serve as a novel approach for clinical treatment of glomerulonephritis, although the specific therapeutic dosage of compound Sanqi granules and P. fallax Hemsl requires further investigation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Xusheng Liu https://orcid.org/0000-0002-2763-8974
References
- 1.Wieliczko M, Dylewska M. [IgA nephropathy - prognostic factors and treatment]. Wiad Lek 2016; 69: 707–710. [PubMed] [Google Scholar]
- 2.Hughes J, Savill JS. Apoptosis in glomerulonephritis. Curr Opin Nephrol Hypertens 2005; 14: 389–395. [DOI] [PubMed] [Google Scholar]
- 3.Kashgarian M, Sterzel RB. The pathobiology of the mesangium. Kidney Int 1992; 41: 524–529. [DOI] [PubMed] [Google Scholar]
- 4.McAdoo SP, Pusey CD. Anti-glomerular basement membrane disease. Clin J Am Soc Nephrol 2017; 12: 1162–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Müller MB, Hoppe JM, Bideak Aet al. Exclusive expression of transmembrane TNF aggravates acute glomerulonephritis despite reduced leukocyte infiltration and inflammation. Kidney Int 2019; 95: 75–93. [DOI] [PubMed] [Google Scholar]
- 6.Ye H, Su B, Ni Het al. microRNA-199a may be involved in the pathogenesis of lupus nephritis via modulating the activation of NF-kappaB by targeting Klotho. Mol Immunol 2018; 103: 235–242. [DOI] [PubMed] [Google Scholar]
- 7.Jefferson JA. Complications of immunosuppression in glomerular disease. Clin J Am Soc Nephrol 2018; 13: 1264–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfister F, Büttner-Herold M, Amann K. [(Immuno)pathology of drug side effects in the kidney]. Pathologe 2018; 39: 576–582. [DOI] [PubMed] [Google Scholar]
- 9.Li SG, Chen Y, Zhang YQ. [Effects of Astragalus polysaccharide on nephritis induced by cationic bovine serum albumin in rats]. Zhong Yao Cai 2010; 33: 1913–1916. [PubMed] [Google Scholar]
- 10.Zhang GG, Ye RG, Kong QY. [ Effects of radix Salivae miltiorrhizae on proliferation, apoptosis and c-myc protein expression of fibroblast in culture of kidney with lupus nephritis]. Zhongguo Zhong Xi Yi Jie He Za Zhi 1997; 17: 473–475. [PubMed] [Google Scholar]
- 11.Hong D, Bai YP, Shi RZet al. Inhibitory effect of reinioside C on vascular smooth muscle cells proliferation induced by angiotensin II via inhibiting NADPH oxidase-ROS-ENK1/2-NF-kappaB-AP-1 pathway. Pharmazie 2014; 69: 698–703. [PubMed] [Google Scholar]
- 12.Shen P, Yang X, Jiang Jet al. Wedelolactone from Eclipta alba inhibits lipopolysaccharide-enhanced cell proliferation of human renal mesangial cells via NF-κB signaling pathway. Am J Transl Res 2017; 9: 2132–2142. [PMC free article] [PubMed] [Google Scholar]
- 13.Bochu W, Liancai Z, Qi C. Primary study on the application of serum pharmacology in Chinese traditional medicine. Colloids Surf B Biointerfaces 2005; 43: 194–197. [DOI] [PubMed] [Google Scholar]
- 14.Duann P, Ho TY, Desai BDet al. Mesangial cell apoptosis induced by stimulation of the adenosine A3 receptor: signaling and apoptotic events. J Investig Med 2005; 53: 37–43. [DOI] [PubMed] [Google Scholar]
- 15.Watson S, Cailhier JF, Hughes Jet al. Apoptosis and glomerulonephritis. Curr Dir Autoimmun 2006; 9: 188–204. [DOI] [PubMed] [Google Scholar]
- 16.Westhorpe CLV, Norman MU, Hall Pet al. Effector CD4(+) T cells recognize intravascular antigen presented by patrolling monocytes. Nat Commun 2018; 9: 747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo X, Zhang J, Liu Met al. Protective effect of ginsenoside Rg1 on attenuating anti-GBM glomerular nephritis by activating NRF2 signalling. Artif Cells Nanomed Biotechnol 2019; 47: 2972–2979. [DOI] [PubMed] [Google Scholar]
- 18.Wang YJ, He LQ, Sun Wet al. Optimized project of traditional Chinese medicine in treating chronic kidney disease stage 3: a multicenter double-blinded randomized controlled trial. J Ethnopharmacol 2012; 139: 757–764. [DOI] [PubMed] [Google Scholar]
- 19.Xu KP, Huang W, Tan JBet al. [ Study on the antihyperlipidemia effective constituent of Polygala fallax Hemsl]. Zhong Yao Cai 2006; 29: 16–19. [PubMed] [Google Scholar]
- 20.del Nogal M, Troyano N, Calleros Let al. Hyperosmolarity induced by high glucose promotes senescence in human glomerular mesangial cells. Int J Biochem Cell Biol 2014; 54: 98–110. [DOI] [PubMed] [Google Scholar]
- 21.Miller CG, Pozzi A, Zent Ret al. Effects of high glucose on integrin activity and fibronectin matrix assembly by mesangial cells. Mol Biol Cell 2014; 25: 2342–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zoja C, Abbate M, Remuzzi G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dial Transplant 2015; 30: 706–712. [DOI] [PubMed] [Google Scholar]
- 23.Esper RJ, Nordaby RA, Vilarino JOet al. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol 2006; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murphy JM, Jeong K, Rodriguez YARet al. FAK and Pyk2 activity promote TNF-alpha and IL-1beta-mediated pro-inflammatory gene expression and vascular inflammation. Sci Rep 2019; 9: 7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roque M, Fallon JT, Badimon JJet al. Mouse model of femoral artery denudation injury associated with the rapid accumulation of adhesion molecules on the luminal surface and recruitment of neutrophils. Arterioscler Thromb Vasc Biol 2000; 20: 335–342. [DOI] [PubMed] [Google Scholar]
- 26.Shen W, Anwaier G, Cao Yet al. Atheroprotective mof Tilianin by inhibiting inflammation through down-regulating NF-kappaB pathway and foam cells formation. Front Physiol 2019; 10: 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang GG, Bai YP, Chen MFet al. Asymmetric dimethylarginine induces TNF-alpha production via ROS/NF-kappaB dependent pathway in human monocytic cells and the inhibitory effect of reinioside C. Vascul Pharmacol 2008; 48: 115–121. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Li C, Li Set al. Dihydrotanshinone I alleviates crystalline silica-induced pulmonary inflammation by regulation of the Th immune response and inhibition of STAT1/STAT3. Mediators Inflamm 2019; 2019: 3427053. [DOI] [PMC free article] [PubMed] [Google Scholar]