Table 2.
Nucleobase modifications and their effects on PNA backbone.
| S. No. | Modified Nucleobase | Structure | Properties | References |
|---|---|---|---|---|
| 1. | 2,6-Diamino purine | 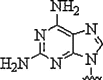 |
Increased affinity and selectivity for thymine | (Haaima et al., 1997) |
| 2. | Pseudoiso-cytosine | 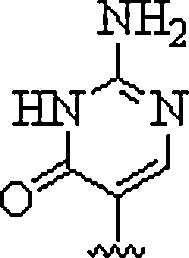 |
Mimics the C+ recognition pattern for triplex formation irrespective of surrounding pH | (Haaima et al., 1997) |
| 3. | 2-Amino purine | 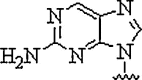 |
Can hydrogen bond with uracil and thymine in the reverse Watson-Crick mode and being inherently fluorescent, can be used to study the kinetics of the hybridization process with complementary nucleic acids | (Gangamani and Kumar, 1997) |
| 4. | Thiazole | 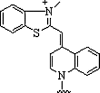 |
Forms PNA probe that fluoresced upon hybridization | (Köhler and Seitz, 2003, Jarikote et al., 2005) |
| 5. | Hypoxanthine | 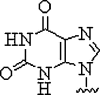 |
Form Watson-Crick base pairs with adenine, cytosine, thymine, and uracil and achieve multimutant specificity | (Sanders et al., 2013) |
| 6. | Thiouracil | 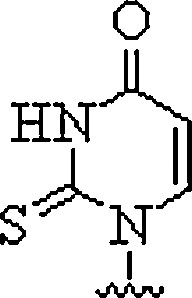 |
Can invade dsDNA in antigene applications | (Haaima et al., 1997) |
| 7. | N4-benzoyl-cytosine | 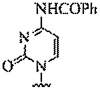 |
candidate for a G-C pseudo-complementary base pair | (Olsen et al., 2011) |
| 8. | 6-Thioguanine | 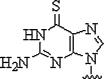 |
Decrease in Tm of 8.5 °C due to PNA:DNA heteroduplex | (Henrik et al., 1999) |
| 9. | G-clamp | 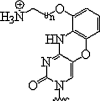 |
Enhanced duplex stability | (Rajeev et al., 2002, Ortega et al., 2007) |
| 10. | P-base | 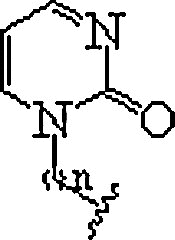 |
In polypurine tracts of double helical RNA, able to isolate pyrimidine interruptions | (Li et al., 2010, Rozners, 2012) |
| 11. | E-base | 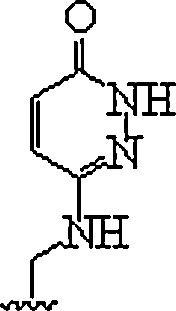 |
In polypurine tracts of double helical RNA, able to isolate pyrimidine interruptions | (Li et al., 2010, Rozners, 2012) |
| 12. | 5(acridin-9-ylamino)uracil | 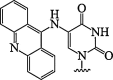 |
Hydrolytically labile modification | (Matarazzo et al., 2013) |
| 13. | Thio-pseudo isocytosine | 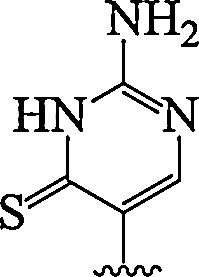 |
Enhanced RNA duplexes recognition | (Devi et al., 2014) |
| 13. | 2-amino pyridine | 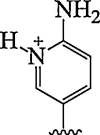 |
Selective triplex formation with RNA duplexes (Adenosine to inosine editing) | (Annoni et al., 2016) |
| 14. | Mono-m-(guanidinoethoxy)phenyl]pyrrolocytosine | 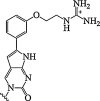 |
Enhanced binding affinity for RNA and an exceptionally high fluorescence quantum yield. | (Wojciechowski and Hudson, 2009) |
