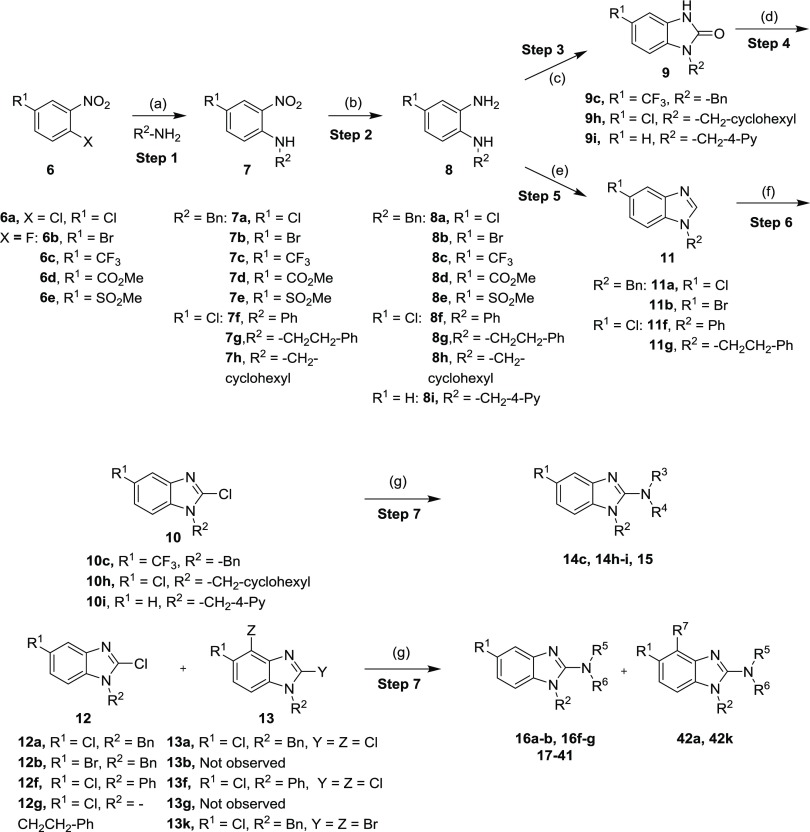
Scheme 1. General Synthetic Approach to the Synthesis of 2-Amino Benzimidazole Derivatives.
Reagents and conditions: (a) Et3N, acetonitrile (ACN), 50 °C, 16 h (56–97%) or K2CO3, dimethylformamide (DMF), 80 °C, 4–18 h (25–98%) or K2CO3, dimethyl sulfoxide (DMSO), 120 °C, 24 h, (93%); (b) Pt/C, H2 balloon, RT, MeOH, 8 h to 3 days (88–97%) or Fe powder, sat. aqu. NH4Cl, EtOH, 90 °C, 6–18 h (69–97%) or NH2NH2·H2O, MeOH, 80 °C, 2 h (61%); (c) triphosgene, dichloromethane (DCM), 25 °C, 16 h (68–94%); (d) POCl3, HCl, 150 °C, 4–24 h, (44–51%) or POCl3, PCl5, 110 °C, 1 h (45%); (e) CH(OMe)3, HCOOH, 100 °C 1–2 h (30–85%) or CH(OEt)3, para-toluene sulfonic acid (PTSA), tetrahydrofuran (THF), reflux, 2 h (88%); (f) lithium diisopropylamide (LDA), Cl3C–CCl3, THF, −78 °C, 4–5 h (33–96%) or LDA, CBr4, THF, −78 °C, 3 4 h (41%); (g) R3R4NH, Et3N, t-BuOH, 120 °C, 6 h to 18 days (9–91%).