Figure 2.
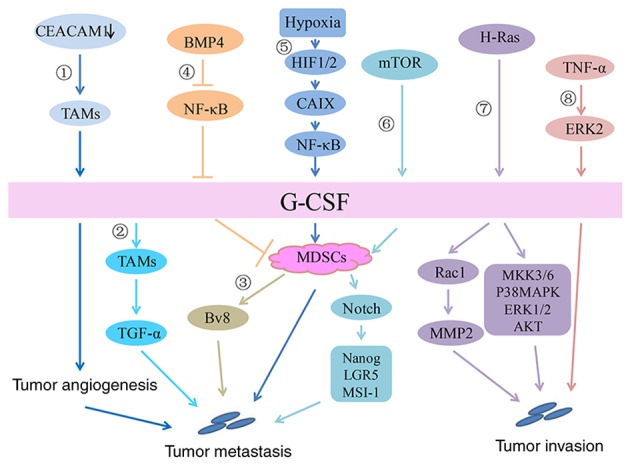
Signaling pathway of G-CSF in breast cancer. In the breast cancer microenvironment: 1) CEACAM1 downregulation promotes G-CSF secretion by TAMs, thereby promoting tumor angiogenesis and initial tumor establishment. 2) By acting on G-CSFR on TAMs, G-CSF increases transforming growth factor-α secretion to promote tumor cell migration. 3) G-CSF increases Ly6G+Ly6C+ granulocytes, which are a type of MDSC and further promotes the production of the proangiogenic factor Bv8 to enhance breast tumor metastasis. 4) BMP4 inhibits the expression and secretion of G-CSF by inhibiting NF-κB, resulting in decreases in the number and activity of MDSCs. 5) In a hypoxic environment, HIF1/2 upregulates CAIX and increases G-CSF expression by activating the NF-κB signaling pathway, which then promotes the mobilization of MDSCs and eventually leads to the lung metastasis of breast cancer. 6) Activation of the AKT-mTOR signaling pathway increases G-CSF expression in tumor cells, thereby promoting the accumulation of MDSCs. MDSCs promote the expression of stem-associated genes, including Nanog, LGR5 and MSI-1, in cancer cells via Notch signaling to promote tumor progression. Direct effect of G-CSF on breast cancer: 7) Stable expression of G-CSF induced by H-Ras upregulates the expression of MMP-2 by activating Rac 1 and promotes the migration/invasion of breast epithelial cells. In addition, overexpression of G-CSF activates other signaling pathways, including MKK3/6, p38 MAPK, ERK1/2 and AKT, thus promoting an invasive phenotype in breast epithelial cells. 8) TNF-α promotes the expression of G-CSF by activating the ERK2 signaling pathway to promote tumor invasion. TNF, tumor necrosis factor; AKT, protein kinase B; G-CSF, granulocyte-colony stimulating factor; HIF, hypoxia inducible factor; MAPK, mitogen associated protein kinase; NF, nuclear factor; mTOR, mammalian target of rapamycin; MDSC, myeloid-derived suppressor cell; CAIX, carbonic anhydrase IX; MMP, matrix metalloproteinase; ERK, extracellular signal regulated kinase; TAM, tumor-associated macrophages; CEACAM1, carcinoembryonic antigen-related cell adhesion molecule 1.
