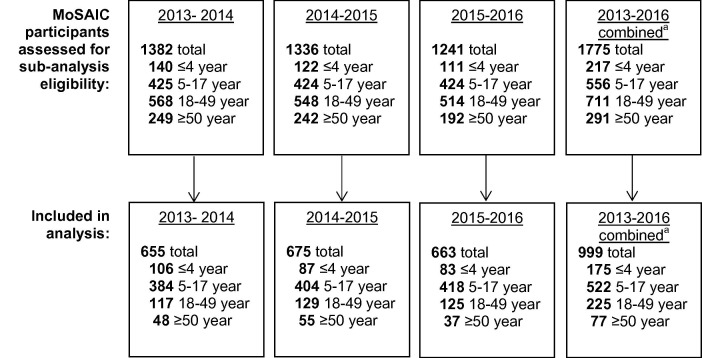
Fig. 2.
Study flow diagram. Eligible children were 6 months to 17 years, had updated vaccine records in the hospital and/or the New York Citywide Immunization Registry (CIR); children who received two doses of influenza vaccine were excluded from the analysis of the season in which they received two doses. Eligible adults were ≥18 years, were patients at the affiliated hospital, and had ≥1 primary care visit or hospitalization between October 1 and the end of the study season of interest.a Combined totals do not equal sum of years as participants may have been active in the study for more than one year. Age classification for combined years is based on age on September 1, 2013.