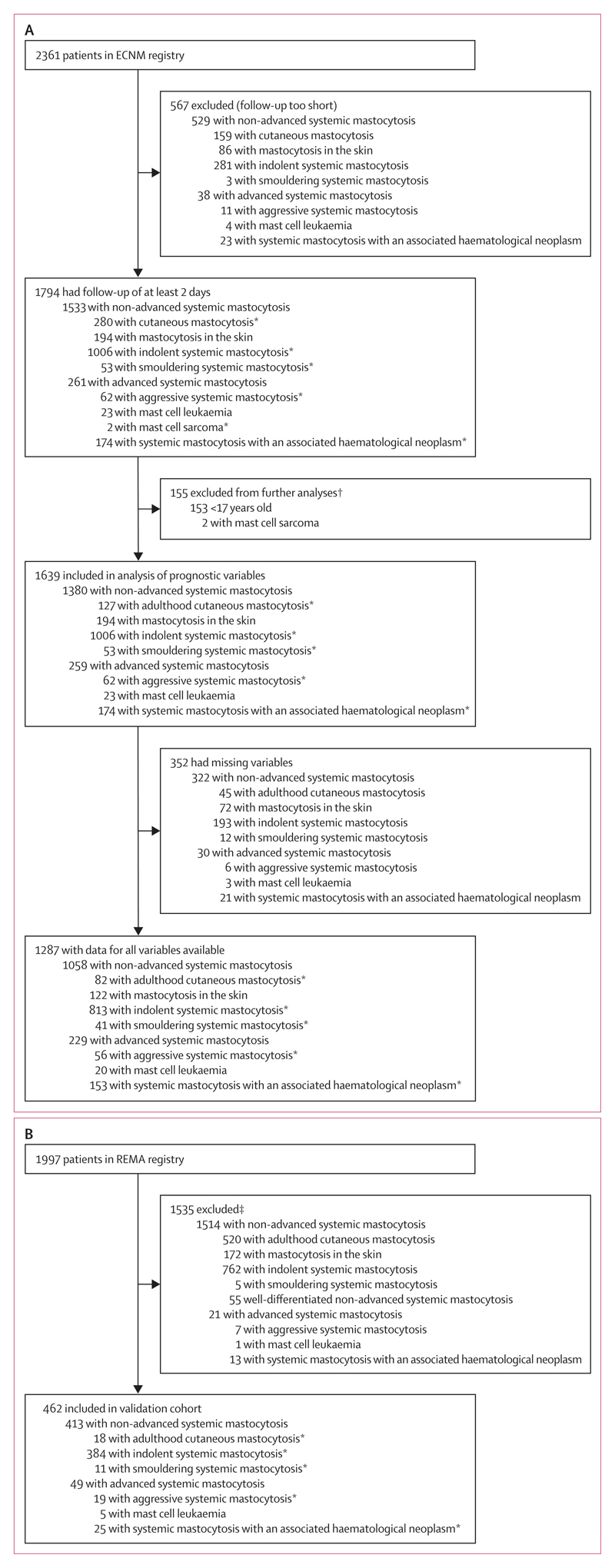
Figure 1. Study profile.
Patients were selected from the ECNM registry (A) and REMA (B). In the ECNM cohort, only patients with at least 2 days of follow-up were included. All patients were included in analyses of overall survival and event-free survival. ECNM=European Competence Network on Mastocytosis. REMA=Red Española de Mastocitosis. *Included in analyses of progression-free survival. †Children (aged <17 years) were excluded from the assessment of prognostic factors and development of the score because, for most children, no bone marrow studies were available and because the disease is different. Patients with mast cell sarcoma were excluded because of the rarity of the disease and its unique pathology and pathogenesis. ‡Those excluded were children (aged <17 years), had cutaneous mastocytosis without a bone marrow study, had less than 12 months of follow-up for non-advanced systemic mastocytosis, or did not have enough data.