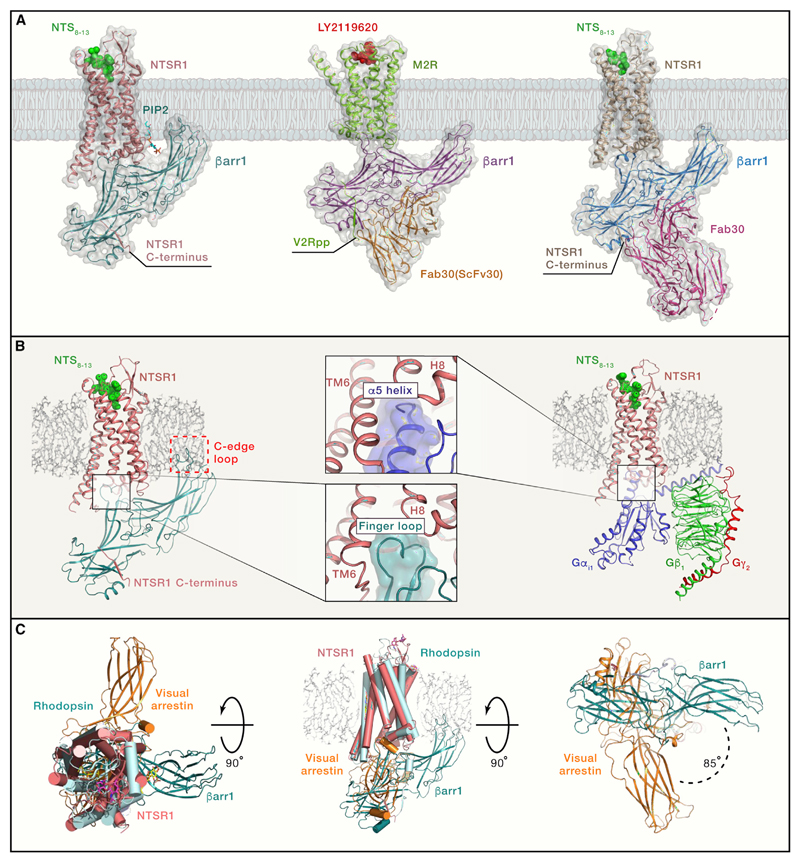
Figure 1. Structural Snapshots of GPCR-βarr1 Complexes.
(A) Thecryo-EM structures of the NTSR1-βarr1 (left panel; PDB: 6UP7), M2R-βarr1-Fab30(ScFv30) (middle panel; PDB: 6U1N), and NTSR1-βarr1-Fab30 (right panel; PDB: 6PWC). The NTSR1-βarr1 structure by Huang et al. (2020) uses a cross-linked complex, whereas the other structure determined by Yin et al. (2019) uses NTSR1-βarr1 fusion protein stabilized by Fab30. The M2R-βarr1 complex has a chemically ligated phosphopeptide (V2Rpp) at the M2R carboxyl terminus and is further stabilized by Fab30 (ScFv30).
(B) The left panel shows the NTSR1-βarr1 in a reference bilayer to indicate the membrane contact of the C edge of βarr1 (red dotted box), a feature observed in all three structures. The right panel shows a cryo-EM structure of the NTSR1-G-protein complex (PDB: 6OS9). The middle panels indicate a similar docking interface of βarr1 finger loop and the α5 helix of Gαi in the respective structures, a feature that is also apparent upon comparison of M2R-βarr1 and M2R-Gαo structures.
(C) Superimposition of the NTSR1-βarr1 structure (PDB: 6UP7) with the crystal structure of rhodopsin-visual arrestin complex (PDB: 5W0P) reveals differential orientation of βarr1 in the membrane plane. The left panel shows the top view from the extracellular side, the middle panel shows the side view, and the right panel shows the bottom view from the intracellular side. This rotation of βarr1 is also observed in the NTSR1-βarr1-Fab30 structure but not in the M2R-βarr1-Fab30(ScFv30) structure.