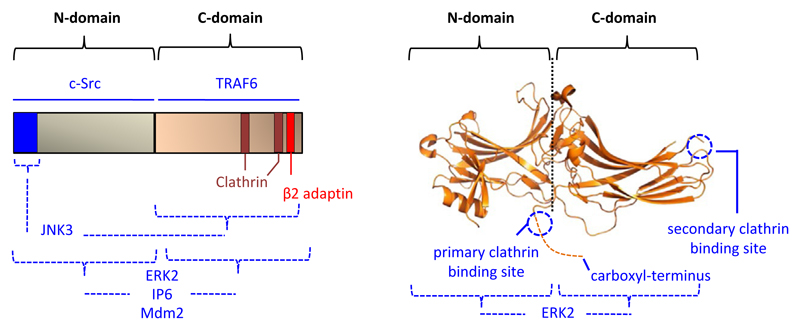
Fig. 2.
Structural representation of β-arrestin indicating some of the interaction interfaces. A potential mechanism underlying the multi-functionality of β-arrestins is their ability to interact with a large number of cellular partners. As some of the interaction interfaces are quite distinct from each other, it offers a framework to modulate selected β-arrestin functions by targeting the protein-protein interaction. This image is adopted from and modified based on a previous publication from our laboratory Ghosh, E., Srivastava, A., Baidya, M., Kumari, P., Dwivedi, H., Nidhi, K., et al. (2017). A synthetic intrabody-based selective and generic inhibitor of GPCR endocytosis. Nature Nanotechnology, 12, 1190–1198.