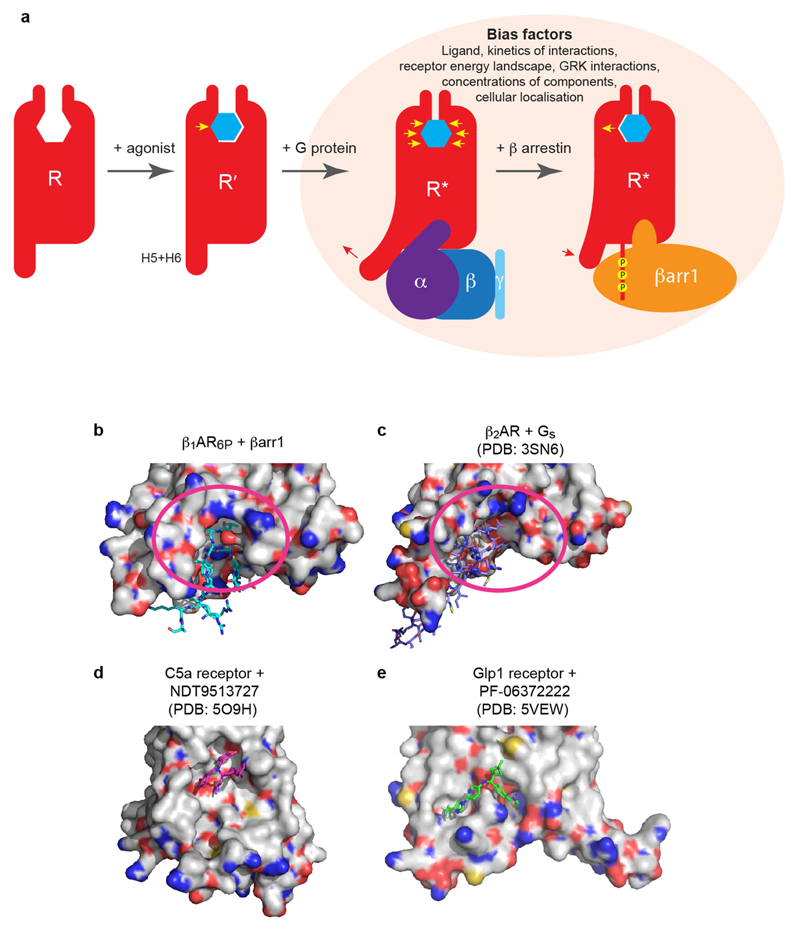
Extended Data Fig. 9. Conformational changes in β1AR and potential drug-interaction sites to discriminate between complexes of β-adrenoceptors coupled to either βarr1 or Gs.
a, The inactive state (R) of β1AR binds agonist (blue hexagon) resulting in an inward movement of H5 in the orthosteric binding pocket (yellow arrow), to form an intermediate state (R′). Coupling of G protein results in outward movement of the cytoplasmic ends of H5 and H6 (red arrow) and contraction of the orthosteric binding site (yellow arrows). Displacement of G protein by arrestin results in an inward movement of the cytoplasmic ends of H5 and H6 (red arrow) and an outward movement of H5 in the orthosteric biding pocket (yellow arrow). Receptors in the R* state have higher affinity for agonists than those in the R state. Representative structures of each of the states depicted have been determined, but in reality there is likely to be a continuum of states between them. Several factors probably affect the arrestin bias of ligands, not just the structure of the receptor–arrestin complex. b, Surface view of β1AR6P showing the finger loop of βarr1 (sticks). c, Surface view of β2AR showing the α5 helix of Gs (sticks). In b, c, potential druggable sites are depicted (magenta oval) in b and c that could be used to develop small molecules that discriminate between the same receptor coupled to either βarr1 or Gs. d, e, Two examples of small-molecule negative allosteric modulators that bind to the surface of GPCRs, which give a proof of concept to the surface-interacting molecules.