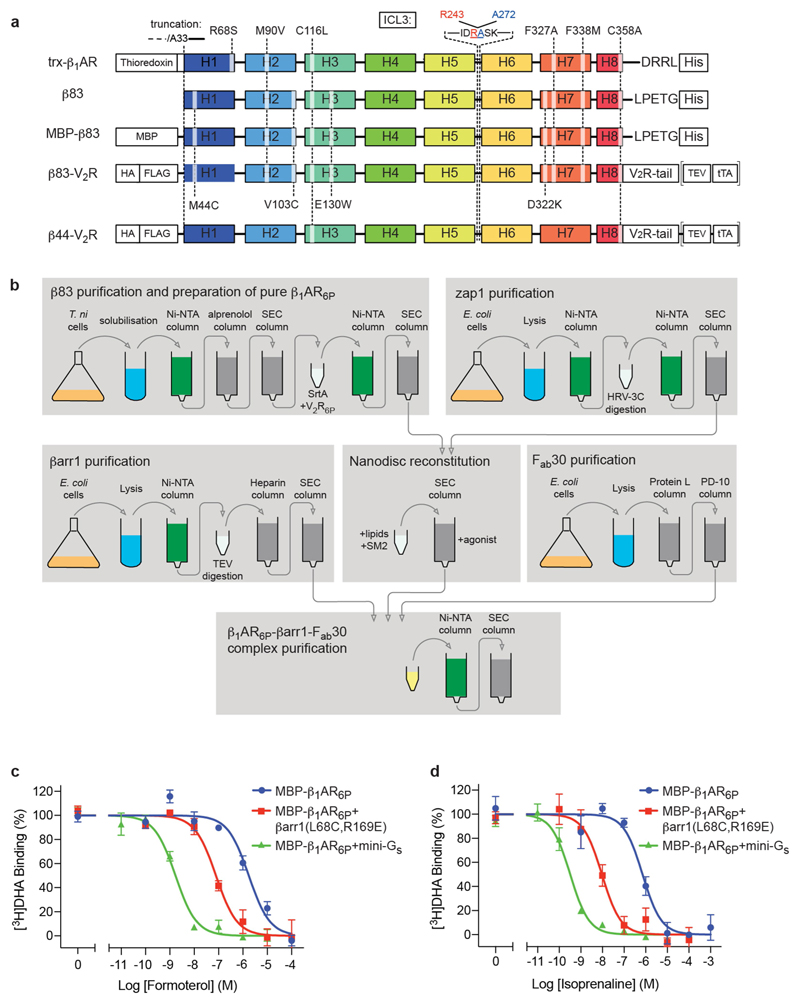
Extended Data Fig. 1. β1AR constructs, purification and activity.
a, Schematic of the constructs used for X-ray crystallography (trx-β1AR), cryo-EM (β83), radioligand binding (MBP-β83) and cell-based assays (β44–V2R, β83–V2R), indicating the sites of truncations, point mutations and tags. b, Purification scheme for the preparation of the β1AR6P–βarr1–Fab30 complex for structure determination by cryo-EM. c, d, Representative competition binding curves using either formoterol (c) or isoprenaline (d) show the high-affinity state of MBP–β1AR6P stabilized by either mini-Gs or βarr1. Experiments (Methods) to determine the high-affinity state were performed in a molar excess of mini-Gs (green curves; n = 2) or βarr1 (red curves; n = 3 with formoterol, n = 4 with isoprenaline) and compared to the low-affinity state (blue curves; n = 4). Experiments were all performed in duplicate, with the number of independent experiments indicated (n). Data are mean ± s.e.m. The apparent K i values were determined using the Cheng–Prusoff equation, using apparent K d values for [3H]DHA of 6 nM (MBP–β1AR6P and MBP–β1AR6P + βarr1) and 1.5 nM (MBP–β1AR6P + mini-Gs). K i values for formoterol are 1.5 ± 0.4 µM (MBP–β1AR6P), 42 ± 18 nM (MBP–β1AR6P + βarr1) and 0.7 ± 0.1 nM (MBP–β1AR6P + mini-Gs). K i values for isoprenaline are 340 ± 70 nM (MBP–β1AR6P), 4.4 ± 0.8 nM (MBP–β1AR6P + βarr1) and 0.13 ± 0.02 nM (MBP–β1AR6P + mini-Gs).