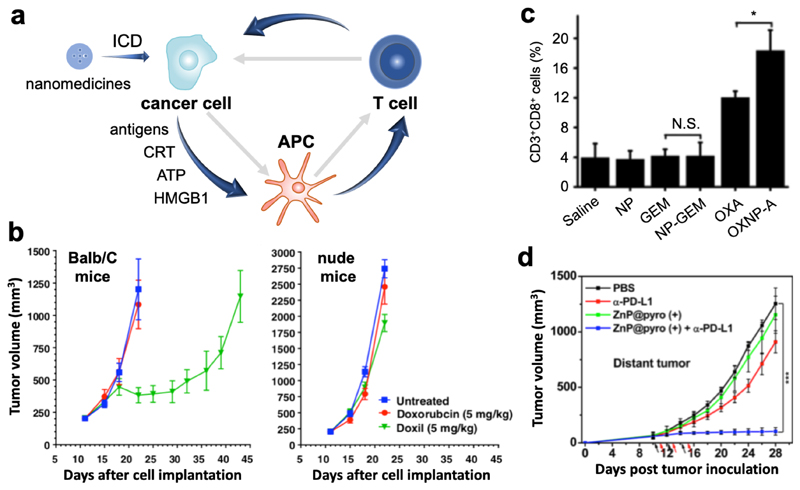
Figure 3. Nano-immunotherapy based on targeting cancer cells.
a: The cancer-immunity cycle is often impaired in tumors (depicted by grey arrows). Upon nanomedicine-based targeting of immunogenic cell death (ICD) inducing drugs to cancer cells, tumor antigens and damage-associated molecular patterns (i.e. calreticulin, adenosine triphosphate and high mobility group box 1 protein) are released. Together, these components promote antigen uptake, processing and presentation by antigen-presenting cells, which contributes to the generation of cytotoxic T cells. b: Immunopotentiation exerted by ICD-inducing nanomedicines, such as PEGylated liposomal doxorubicin (Doxil), can be exemplified by the notion that Doxil is significantly more effective in inhibiting CT26 tumor growth in immunocompetent Balb/C mice than in immunodeficient nude mice. The benefit of nanomedicine-based drug targeting to cancer cells was demonstrated by the higher efficacy and lower toxicity of Doxil as compared to free doxorubicin. Adapted with permission from ref. 25. Copyright 2015 Elsevier. c: Nanoparticle-based delivery of ICD-inducing oxaliplatin resulted in stronger immunopotentiation that oxaliplatin in free form, as exemplified by higher levels of CD8+ T cells infiltration into Pan02 tumors. In case of non-ICD-inducing gemcitabine treatment, no enhanced T cell infiltration into tumors was observed. Adapted with permission from ref. 26. Copyright 2016 Elsevier. d: Pyrolipid-loaded nanomedicines induced ICD and potentiated cancer immunity in distant tumors via the abscopal effect, which synergized with anti-PD-L1 antibodies to efficiently eradicate the tumors. Adapted with permission from ref. 27. Copyright 2013 American Chemical Society.