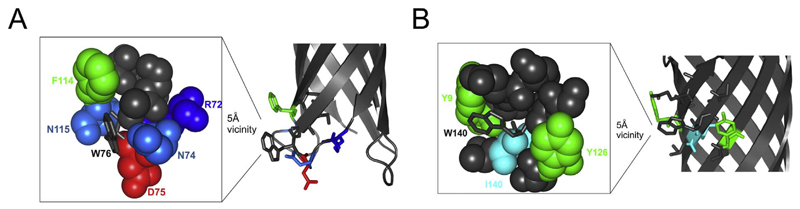
Fig. 5.
Cartoon representation of OmpX highlighting residues in the vicinity of Trp76 and Trp140. (A) The 5 Å vicinity of Trp76 (shown as grey sticks in the magnified image on the left) is populated with polar residues. Here acidic amino acids are shown as red, basic residues as different shades of blue and the aromatic residue is shown as green. Other apolar residues are shown as grey spheres. (B) The 5 Å vicinity of Trp140 (shown as grey sticks in the magnified image on the left) is populated with aromatic, hydrophobic and polar residues. The vicinity of Trp140 does not contain any charged residues. Here, aromatics are shown as green, hydrophobic as cyan and other apolar residues as grey spheres.