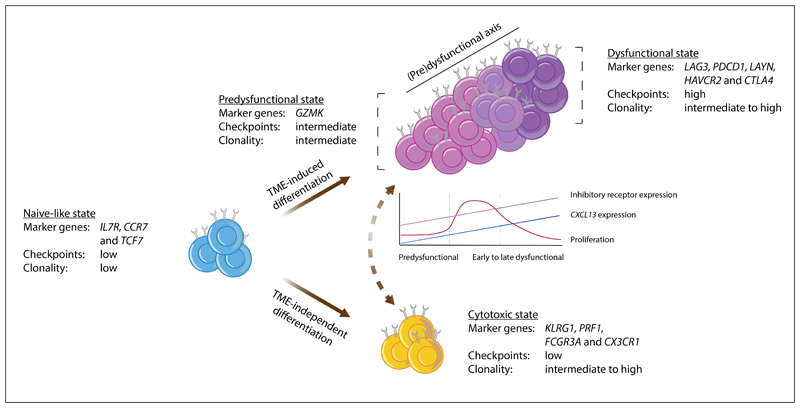
Figure 1. Model of intratumoral CD8+ T cell states.
This schematic depicts a model describing the characteristics of, and possible connections between, the major CD8+ T cell states in human tumors, as based on data from20–22,28–31,33,34. Observations from the different studies that support this model are listed in Supplementary Table S1. In brief, the naïve-like cells described in non-small-cell lung cancer (NSCLC)29, hepatocellular carcinoma (HCC)28, colorectal cancer (CRC)31, basal cell cancer (BCC)30, and melanoma21 show a strong resemblance to the (central-)memory populations described by Sade-Feldman et al. and Clarke et al.22,33. Based on the expression of granzyme K (GZMK), intermediate expression of inhibitory molecules, and relatively low clonality, the T lymphocyte population described in Sade-Feldman et al.22 and the effector–memory population described in Zhang et al.31 were considered similar to the pre-dysfunctional cell states observed in melanoma21, HCC28, and NSCLC29. While additional research is required to determine their extent of overlap, the tissue resident memory T (TRM) population described in triple-negative breast cancer (TNBC)34 and the HAVCR2+ TRM cells in NSCLC33 are here aligned with the dysfunctional state described in all other studies. The cell state definitions from Azizi et al.32 could not be integrated into the model presented here and the effector–memory subset reported by Savas et al. may be composed of a mixture of pre-dysfunctional and cytotoxic cells, based on the combined expression of GZMK and killer cell lectin-like receptor subfamily G member 1 (KLRG1)34. In this model, we propose that the development of (pre-)dysfunctional cell states is predominantly driven by tumor-specific cues such as tumor antigen recognition and/or tumor-specific environmental factors (here referred to as tumor microenvironment (TME)-induced differentiation). Cytotoxic cell states are also encountered in healthy tissues28,29,31, indicating that the underlying differentiation process is not strictly tumor-specific (here referred to as TME-independent differentiation). Cytotoxic effector T cells are depicted as a population that is most likely developmentally distinct from the cells along the (pre-)dysfunctional axis, but additional research is required to clarify whether cytotoxic effector cells indeed originate from a distinct pool of cells, or whether they are connected to the (pre-)dysfunctional axis in some situations (as depicted by the dashed two-way arrow). Note that both trajectory and T cell receptor (TCR) sharing analyses indicate that the pre-dysfunctional and dysfunctional cells form a continuum of cell states, rather than well-demarcated populations. The line graph shows approximate levels of proliferation, CXC-chemokine ligand 13 (CXCL13) expression, and the expression of inhibitory receptors by pre-dysfunctional, early dysfunctional, and late dysfunctional CD8+ T cells. CCR7, CC-chemokine receptor 7; CX3CR1, CX3C chemokine receptor 1; CTLA4, cytotoxic lymphocyte-associated antigen 4; FCGR3A, Fcγ receptor IIIA; IL7R, interleukin 7 receptor; LAG3, lymphocyte activation gene 3; PDCD1, programmed cell death 1; PRF1, perforin 1; TCF7, transcription factor 7.