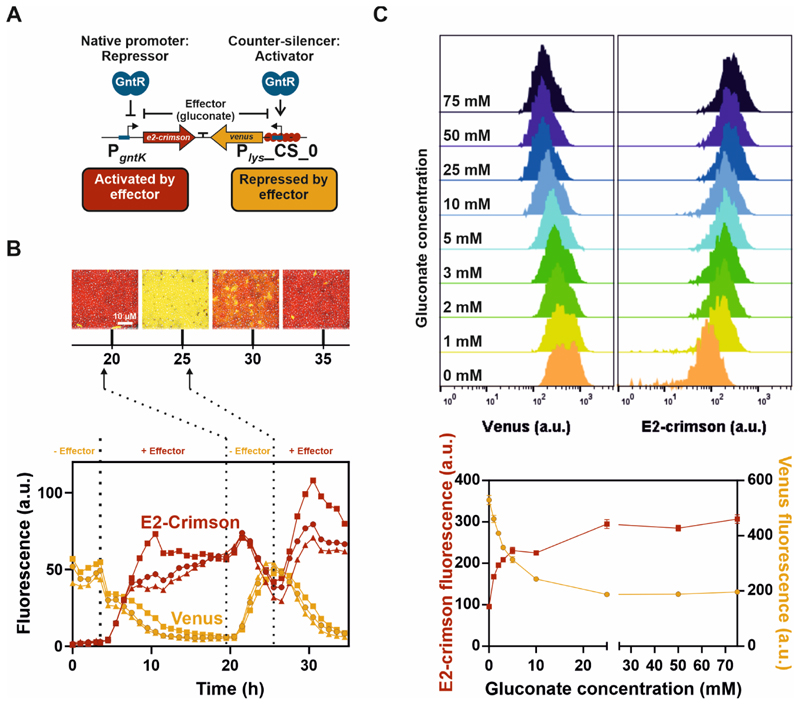
Figure 5. Reversibility and graduated responsiveness of the GntR-dependent toggle switch.
A) Schematic overview of the GntR-dependent toggle switch (adapted from18). B) Reversible switch between both reporter outputs. C. glutamicum wild type cells harbouring the plasmid-based toggle were cultivated in a microfluidic cultivation system65 with continuous supply of CGXII medium supplemented with either 100 mM glucose or 100 mM gluconate and analyzed by time-lapse microscopy at 15 min intervals. Cells were pre-cultivated in shaking flasks in the absence of the effector molecule gluconate (100 mM glucose). After the first 3.5 h (left dotted vertical line) of cultivation in the microfluidic chip in the absence of gluconate, the medium supply was switched to CGXII supplemented with 100 mM gluconate. Further switches of effector supply were performed after 19.5 and 25.5 h (dotted vertical lines). The graphs show the background corrected fluorescence (Venus and E2-Crimson) of three independent microcolonies (circles, squares, triangles) over time and images display one representative colony (triangle) after 20, 25, 30 and 35 h of cultivation. C) Graduated response of the GntR-dependent toggle switch to varying amounts of the effector molecule gluconate. Histograms of Venus (left) and E2-crimson fluorescence (right) after five hours of cultivation of C. glutamicum wild type cells harbouring the plasmid-based toggle. Cultivation was performed in a 96 deep-well plate in CGXII medium supplemented with varying amounts of gluconate and glucose (100 mM carbon source in total) and fluorescence values were analyzed by flow cytometry (FACSAria™ III). Gluconate concentrations are given as numbers. Cells were pre-cultivated in shaking flasks in the absence of the effector molecule gluconate (CGXII with 100 mM glucose). The graph shows the means of heights of fluorescence levels of biological triplicates and error bars the standard deviation.