Summary
Depletion of Wnt signaling is a major requirement for the induction of the anterior prosencephalon. However, the molecular events driving the differential regionalization of this area into eye-field and telencephalon fates are still unknown. Here we show that the BMP pathway is active in the anterior neural ectoderm during late blastula to early gastrula stage in zebrafish. Bmp2b mutants and mosaic loss-of-function experiments reveal that BMP acts as a repressor of eye-field fate through inhibition of its key transcription factor Rx3, thereby protecting the future telencephalon from acquiring eye identity. This BMP-driven mechanism initiates the establishment of the telencephalon prior to the involvement of Wnt antagonists from the anterior neural border. Furthermore, we demonstrate that Rx3 and BMP are respectively required to maintain and restrict the chemokine receptor cxcr4a, which in turn contributes to the morphogenetic separation of eye-field and telencephalic cells during early neurulation.
Introduction
During vertebrate development the rostralmost part of the neural plate differentiates into the forebrain, the most complex region of the central nervous system (Wilson and Rubenstein,2000). Previously, it has been shown that depletion of canonical Wnt signaling is required for the induction of anterior neural fates, including telencephalon, hypothalamus, and eye field (Heisenberg et al., 2001; Houart et al., 2002; Kiecker and Niehrs, 2001; Mukhopadhyay et al., 2001; Nordström et al.,2002). Inside this anterior forebrain territory, the mechanism by which the telencephalon and eye-field fates are established is still unknown. The eye field develops medio-laterally, whereas the telencephalon is located at the rostral margin surrounding the eye field laterally and anteriorly. A graded radial signal, different from Wnt antagonists, secreted along the neural plate border is well suited to induce a horseshoe-shaped telencephalon at the margin versus the eye field at more medial position. One such signal is the Bone Morphogenetic Protein (BMP), which is expressed in the nonneural ectoderm surrounding the neural plate both laterally and anteriorly throughout gastrulation (Barth et al., 1999; Nguyen et al., 1998; Wilson and Houart, 2004).
According to the “BMP default model,” the nascent ectoderm is specified by a readout of specific levels of BMP signaling that gives rise to either nonneural or neural ectoderm (Hemmati-Brivanlou and Melton, 1997; Sasai and De Robertis, 1997; Wilson and Hemmati-Brivanlou, 1997). High levels of BMP activity generate epidermis, whereas intermediate levels induce neural crest (Schumacher et al., 2011; Tribulo et al., 2003) and confer competence to form placodes (Glavic et al., 2004 Kwon et al., 2010). Although BMPsignaling during neural crest specification has been studied extensively (Klymkowsky et al.,2010) and evidence for BMP-controlled placodal establishment is accumulating (Bailey and Streit, 2006; Patthey and Gunhaga,2011; Schlosser et al., 2008), the impact of early BMP signaling on neural plate fates is still debated.
Studies in Xenopus and chick have shown that neural induction requires early FGF signaling in addition to BMP inhibition (Delaune et al., 2005; Linker and Stern, 2004; Streit et al.,2000). In zebrafish, levels of BMP activity have been shown to define identities along the neural plate medio-lateral axis, the future dorsoventral (D-V) axis of the CNS (Barth et al., 1999; Langdon and Mullins, 2011 Tucker et al., 2008). In BMP-depleted embryos (swr ta72−/−, mutants carrying a null mutation for bmp2b) and noggin-injected embryos, respectively, neural plate markers are expanded along the medio-lateral axis, with the exception of the telencephalon, which is reduced or completely absent (Barth et al., 1999; Houart et al., 2002). positioned sensory neurons are also absent in swr ta72−/−, whereas more medially located interneurons are expanded, indicating a functional decreasing gradient of BMP activity from marginal to medial fates in the neural plate (Barth et al., 1999 Nguyen et al., 2000). In addition to the key role of BMP in establishing D-V polarity during late blastula and gastrula stages, its influence upon anteroposterior (A-P) patterning was suggested by transplantation of noggin-expressing cells in the nonneural ectoderm near the anterior neural border (ANB) in zebrafish (Houart et al., 2002). These cells induce ectopic expression of telencephalic marker at a defined distance from the graft. Hence, the most anterior neural (future telencephalic) tissue seems to be induced by a specific threshold of BMP signals.
The role of a morphogen gradient is not only to provide posi-tional information to a given cell but also to regulate precise timing of specification. The positional information that is pro-vided by a graded signal is not static because it is distorted over time by morphogenetic movements and can be modified by other intracellular modulators. As for the BMP-driven patterning of the D/V axis, it has been shown that cells respond to the gradient in a time-dependent manner, according to their position along the A/P axis (Tucker al., 2008).
Our data reveal that a threshold of BMP activity inside the anterior neural ectoderm (prospective telencephalon) is required to inhibit expression of the transcription factor Rx3. Rx3 in zebrafish, and its homolog Rx/Rax in mouse, has been shown to be a key component of eye-field induction (Loosli et al.,2003; Mathers et al., 1997), and Rx3 mutants revealed that it acts as a potent repressor of telencephalic identity (Stigloheret al., 2006). The required graded BMP information is tightly temporally controlled and limited to late blastula to early gastrulation stages. By late gastrula, telencephalon and eye field have to adopt very distinct morphogenetic movements to orchestrate initiation of prosencephalic neural plate closure and formation of the eye vesicles. Early specification of telencephalon and eye fates is therefore essential to avoid cell mixing of these two populations during forebrain morphogenesis. We provide evidence that a key role of the early BMP activity inside the ANB is to segregate telencephalic from eye-field progenitors through regulation of cxcr4a. Expression of this chemokine receptor is maintained by Rx3 in the eye field and repressed by BMP activity inside the prospective telencephalon. This restricted expression contributes to telencephalon/eye boundary formation, through promoting cohesion inside the eye-field population during early neurulation.
Results
Telencephalic Fate Is Lost, and the Eye Field Is Expanded in bmp2b Mutants
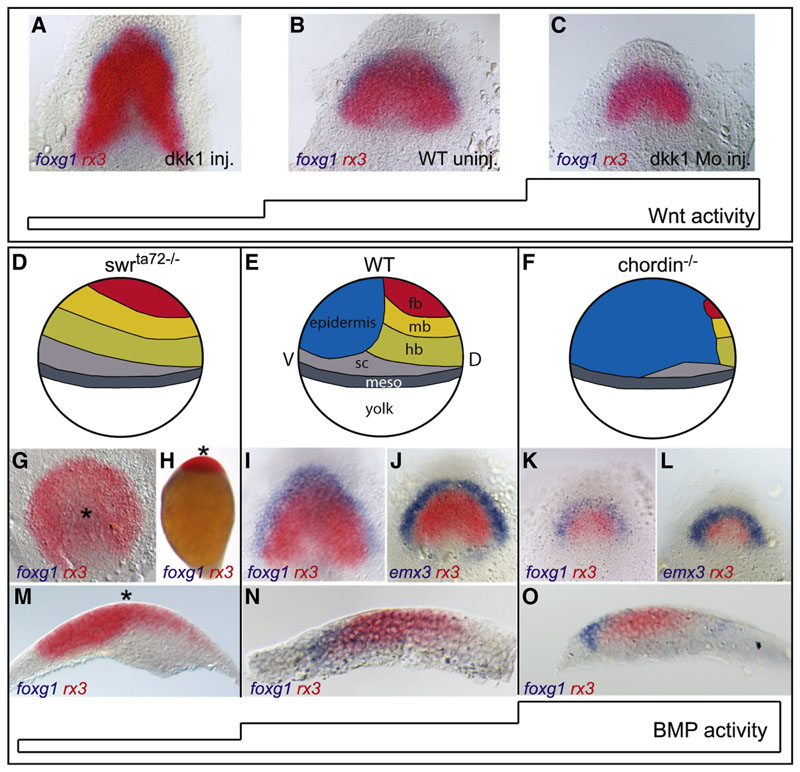
A prerequisite to early forebrain develop-ment is the depletion of Wnt activity within the anterior neural plate (Heisenberg et al., 2001; Houart et al., 2002; Kiecker and Niehrs, 2001). Manipulation of Wnt activity by injection of the Wnt antagonist dickkopf (dkk1) and dkk1 morpholino gives rise to an expansion and reduction of the nascent anterior neural plate, respectively (Figures 1A–1C). These changes affect both the telencephalon and the eye field, leaving the mechanism by which eye and telen-cephalon fates are differentiated unanswered. To test whether the BMP pathway may part telencephalon and eye-field fates, we analyzed the bmp2b null mutant swr ta72−/− which was shown to be devoid of any BMP activity (Figure 1D; Barth et al., 1999; Dick et al., 1999; Mullins et al., 1996 Nguyen et al., 1998), as well as the BMP antagonist mutant chordino (Figure 1F; Hammerschmidt et al., 1996; Schulte-Merker et al.,1997). Foxg1, induced in the horseshoe-shaped telencephalon territory at the rostral margin of the wild-type neural plate at bud stage/end of gastrulation (Figures 1I and 1N), is absent in swr ta72−/− embryos (Figures 1G, 1H, and 1M; see also Barth et al., 1999). In contrast to all other neural markers tested (fkd3, otx2, flh, her5), telencephalic markers (foxg1, fgf8, anf1) are not expanded throughout the ventral ectoderm but are absent or strongly reduced in swr ta72−/− (see Figures S1A–S1F available online; Barth et al., 1999; Nguyen et al., 1998), indicating that BMP activity is required early to establish the telencephalon (Houart et al., 2002). We tested whether the loss of telencephalic identity may be due to transformation into eye precursors. Within wild-type zebrafish anterior neural plate, rx3 transcripts are restricted to the medially located eye-field territory (Figures 1l,1J, and 1N). Upon BMP depletion in swr ta72−/−, rx3 expression is massively expanded and radialized within the entire anterior ectoderm (Figures 1G, 1H, and 1M). The expansion of rx3 in swr ta72−/−is readily detectable at the onset of rx3 expression, around 75% epiboly/midgastrula stage (Figures S1A and S1B).
Figure 1. BMP, but Not Wnt, Subdivides the Anterior Forebrain Neural Fate into Eye and Telencephalon.
(A-C) Foxg1/rx3 in situ hybridization of dkk1 RNA (A) and dkk1 morpholino-injected embryos (C) compared to uninjected control (B) bud stage embryos (end of gastrulation), dorsal view,anterior to the top.
(D-F) Schematic illustration of fate changes in response to BMP depletion in swrsup>ta72 −/− (D) and elevated level of BMP signaling in chordin−/ −(F), compared to wild-type (E) bud stage embryos, lateral view, dorsal to the right. fb, forebrain; mb,midbrain;
hb, hindbrain; sc, spinal chord; meso, mesoderm.
(G-O) Foxg1, rx3 double in situ hybridization in swrsup>ta72 −/− (G, H, and M), WT (I, J, and N), and chordin−/− (K, L, and O). Asterisks in (G), (H), and (M) mark the center of the radialized axis. (G) Animal pole view, (H) lateral view, (I-L) dorsal view, anterior to the top, (M-O) sagittal section of the neural plate, anterior to the left.
See also Figure S1
In contrast, Chordino null mutants exhibit a much smaller neural plate due to increased BMP activity, shown by strong upregulation of Smad1,5,8 phosphorylation on the dorsal side of the embryo at early gastrula stages (compare Figures 1K and 1L to 1I and 1J; Figures S1G and S1H). Anteriorly, expression of eye-field marker rx3 is strongly reduced, whereas the telencephalic marker expression (foxg1, emx3) does not shrink proportionally (compare Figures 1K, 1L, and 1O to 1I, 1J, and 1N) but rather displays an equally wide domain than observed in siblings. These findings suggest that telencephalic identity requires BMP signaling activity, whereas the eye field demands low or no BMP signaling.
BMP Is Required to Pattern the Anterior Margin of the Neural Plate in Pre- and Early Gastrula Stage Embryos
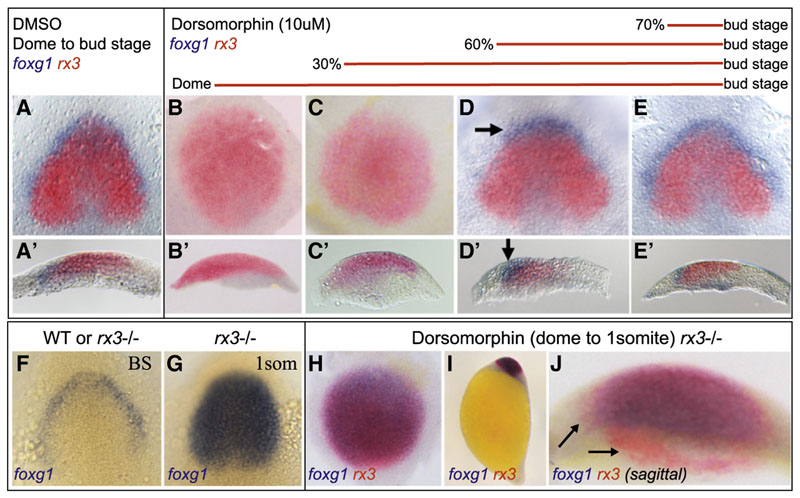
To identify the time window of BMP requirement, we treated embryos with 10 μM of dorsomorphin (DM; Yu et al., 2008) prior to or during gastrulation and assessed forebrain patterning at bud stage by detecting foxg1 and rx3 expression. Administering DM at dome stage (late blastula) or 30% epiboly (very early gastrula) phenocopied the swr ta72−/− mutant (compare Figures 2B and 2C to 1G and 1M).Partial rescue of the most rostral telencephalic domain was observed in embryos incubated with DM from 60%(early to midgastrula) to bud stage (arrow in Figure2D). Slightly later onset of DM treatment (from 70%/mid gastrula stage onward) gave rise to a normally patterned telencephalon and eye field (Figure 2E), compared to DMSO-treated control embryos (Figure 2A). Increasing the dosage of DM to 50 μM showed similar effects on foxg1 and rx3 expression, although the overall expression level of all tested markers appeared weaker, and development was delayed in these embryos (Figure S2).In summary,the entire anteriormost neural plate territory expresses rx3 in absence of BMP, indicating a transformation of the presumptive telencephalon into eye field (Figures 1 and 2). This finding suggests that the BMP pathway promotes telencephalicfate either directly or indirectly by repressing eye-field fate.
Figure 2. Patterning of the Anterior Neural Plate Requires BMP Signaling at Pre- and Early Gastrula Stages.
(A-E) Embryos were treated with 10 μM of DM (B-E) or equivalent volume of DMSO (A) at time points indicated. All embryos were fixed at bud stage followed by double in situ against foxg1 and rx3. (A, D, and E) Dorsalview, (B and C) animalpole view, and (A’-E’) sagittal section. DM treatment from 60% epiboly until bud stage results in partial rescue of the telencephalon at most anterior position (arrows in D and D’).
(F-J) In rx3−/− mutants foxg1 expression is expanded across the eye field at one-somite stage (G), but not at bud stage (F). In DM-treated rx3−/− embryos (H-J), the expanded eye field expresses foxg1 in the entire rx3-positive domain, except at its margin and a small stripe of cells at the medial/ventral position (arrows in J). (F and G) Dorsal view, anterior to the top, (H) animal pole view, (I) lateral view, (J) sagittal section, ventral to the left. BS, bud stage; 1som, one-somite stage.
See also Figure S2.
We therefore tested whether telencephalic fate is established in the absence of BMP signaling in embryos lacking the key eyefield effector rx3. In homozygous rx3 mutant embryos, the eye field is converted into telencephalon at the one-somite stage (Figures 2F and 2G; Stigloher et al., 2006). When treated with DM from dome stage (blastula) onward, rx3 bud stage embryos show a widely expanded rx3-positive domain (null mutants express nonfunctional transcripts) as a result of BMP abrogation. By one-somite stage this domain is almost completely converted into telencephalon (Figures 2H–2J). This demonstrates that BMP signaling is not essential for the acquisition of telencephalic fate. However, it is strictly required to protect the telencephalon from adopting retinal identity through repression of rx3 within a well-defined time window from late blastula to midgastrula stages.
The BMP Pathway Is Active within the Neural Plate during Gastrulation
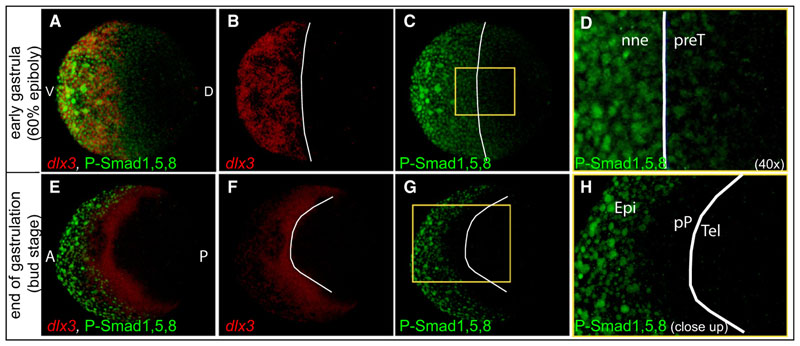
The data above demonstrate that BMP activity is necessary during early gastrulation to prevent expression of the eye effector Rx3in the future telencephalon but did not reveal where and how it acts (directly or indirectly) upon rostral neural plate fates. To address this, we first analyzed the spatial distribution of BMP activity during gastrulation.Using dlx3 expression asa landmark for future nonneural fates (Dutta et al., 2005; Pera and Kessel, 1999), we detected P-Smad1,5,8-positive nuclei inside the anterior neural plate at 60% epiboly/early to midgastrula (Figures 3A–3D). In fact, the four- to fivemost marginal rows of neural plate cells exhibited weak but clearly detectable signal of P-Smad1,5,8 (Figures 3C and 3D), demonstrating the presence of low levels of BMP activity in territories that give rise to anterior neural tissue. At the end of gastrulation, BMP activity recedes from the neural margin, and most of the preplacodal cells as P-Smad1,5,8 slightly overlap with the ventral expression domain of dlx3 (Figures 3E–3H).
Figure 3. BMP Signaling Is Active at the Rostral Margin of the Neural Plate during Early Gastrulation.
(A-H) Whole-mount P-Smad1,5,8 antibody staining (green) and dlx3 transcript detection (red) in 60% epiboly (early gastrula, A-D) and bud stage (end gastrula, E-H).White lines mark the border of dlx3 expression. (A-D) Animal pole view, dorsal to the right. (E-H) Dorsal view, anterior to the left. (D) is a 40 × magnification of the yellow boxed area in (C). (H) is a close-up of the yellow boxed area in (G). All figures are z-projections of confocal sections. nne, nonneural ectoderm; preT, prospective telencephalon; Epi, epidermis; PP, preplacodal territory; Tel, telencephalon. See also Figure S3.
As an additional readout for BMP activity, we used the BMP reporter line (BRE:GFP) (Alexander et al., 2011). Dlx3/BRE:GFP staining at 60% epiboly stage revealed high levels of BMP activity on both sides of the neural plate boundary (Figures S3A–S3C). At late gastrula stages the BRE-GFP gradient has moved ventrally, and the neural plate is BRE-GFP negative (Figures S3D–S3F). Together with results obtained from swr ta72−/− and DM-treated embryos, the P-Smad1,5,8 and BRE-GFP immunostainings revealed a functionallyrelevant level of BMP activity within the nascent marginal neural plate at early to midgastrula stages. However, during the second half of gastrulation, BMP activity declines, and anterior neural cells are unaffected by BMP depletion.
Mosaic Depletion of BMP within the Telencephalon Triggers Ectopic Expression of Eye-Field Marker
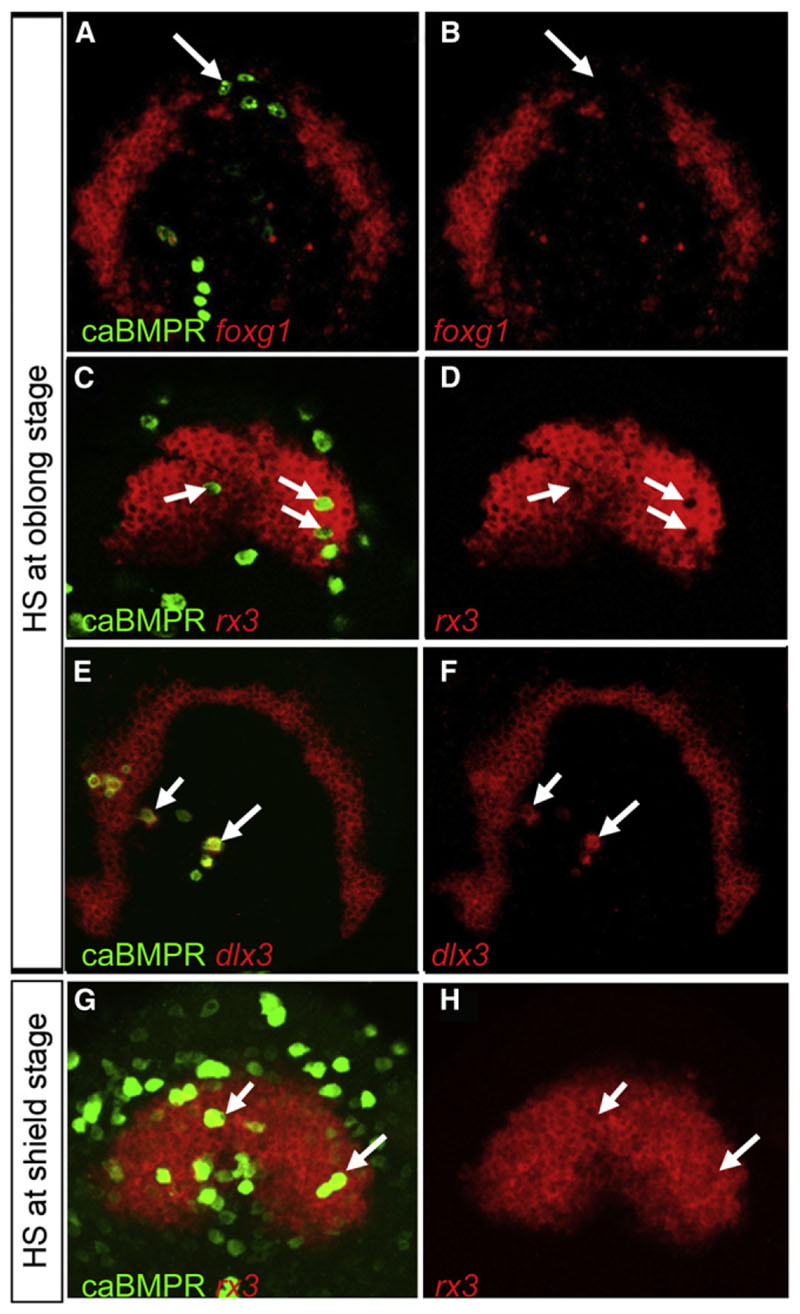
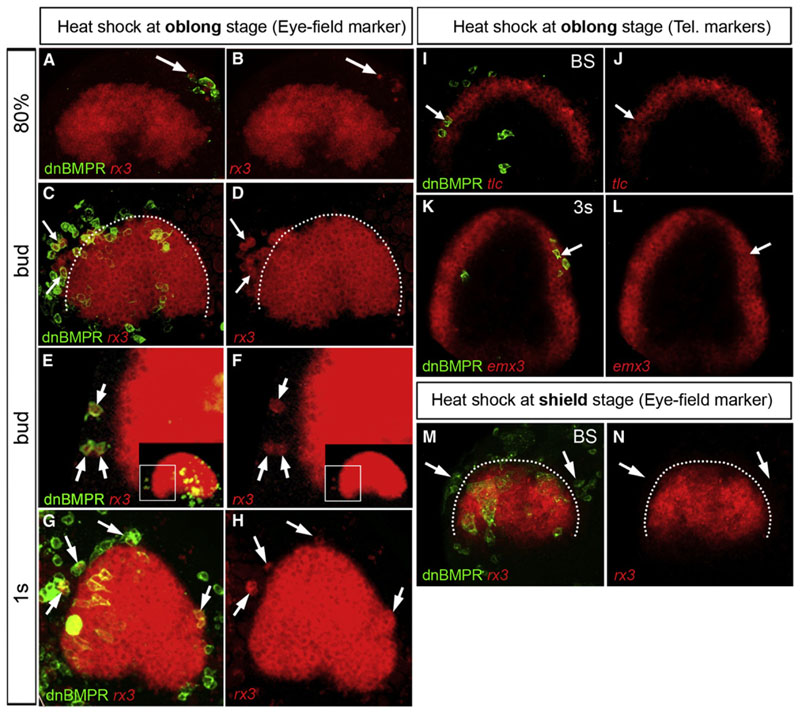
swr ta72−/− and DM-treated embryos clearly show an expansion of the eye field at the expense of the telencephalon, and BMP activity is found inside the rostral neural plate at the time DM treatment influences these rostral fates. We therefore postulated that a threshold of BMP activity is required inside the telencephalic precursors to prevent them from taking an eye fate. We assess this possibility, investigating how single cells behave when they gain or lose BMP activity at different times in a wildtype neural plate environment. We generated transiently transgenic mosaic embryos by using either constitutively active (caBMPr; Figure 4) or dominant-negative (dnBMPr; Figure 5) BMP receptor type 1 vectors, driven by HSP70 promoter (Pyati et al., 2005; Quillien et al., 2011). Heat shock induction of caBMPr transgenic cells at oblong (blastula) stage results in high levels of P-Smad1,5,8 cell autonomously (Figures S4A–S4C). Telencephalic caBMPr-positive cells cause a repression of foxg1 expression in a non-cell-autonomous manner (Figures 4A and 4B), whereas caBMPr-positive eye-field cells lose rx3 expression in a strictly cell-autonomous fashion (Figures 4C and 4D). Complementary to the repression of neural markers in the telencephalon and eye field, caBMPr-expressing cells inside the neural plate induce the nonneural ectoderm marker dlx3 cell autonomously (Figures 4E and 4F). The statistical analysis suggests that the vast majority of caBMPr cells that exhibit repression of rx3 expression are identical to the ones with ectopic dlx3 expression (Figure S4D). If the heat shock is per-formed at 50% epiboly (shield stage)/early gastrula, which results in caBMPr expression from ~65% to 70% epiboly/midgastrula stage onward, it has no effect on rx3 expression (Figures 4G and 4H), indicating a tight temporal control of the BMP influence in the anterior neural plate.
Figure 4. Repression of Anterior Neural Plate Markers by Elevated BMP Activity Is Limited to the Early Stage of Gastrulation.
All embryos were injected with HSP70:caBMPr plasmid at one- to two-cell stage followed by heat shock at oblong/early blastula stage (A-F) or shield/early gastrula stage (G and H) and fixed at bud stage. Heat shock induction of caBMPr at oblong stage represses foxg1 expression non-cell-autonomously (arrows in A and B) and rx3 expression cell autonomously (arrows in C and D). Dlx3 is ectopically induced in caBMPr-expressing cells inside the neural plate (arrows in E and F). Heat shock at shield stage does not affect rx3 expression (arrowsinG and H).All figures are z-projections of confocal sections.See also Figure S4.
Figure 5. Mosaic BMP Loss of Function at Early Stages inside the Telencephalon Results in Ectopic Expression of rx3.
Embryos were injected with HSP70:dnBMPr plasmid at one- to two-cell stage followed by heat shock at oblong stage/early blastula (A-L) and shield stage/early gastrulation (M and N), respectively. The arrows in (A)-(H) mark cells with ectopic rx3 expression at stages indicated. (E and F) Close-up of the boxed area marked in the inset. (I-L) Expression of telencephalic (Tel.) markers, tlc and emx3, is maintained in dnBMPr-expressing cells (arrows in I-L). (M and N) Heat shockat50% epiboly/early gastrulation does not result in ectopic rx3 expression (arrows in M and N). All views are dorsal, anterior at the top. White dotted lines in (C), (D), (M), and (N) mark the border of the eye field. All figures are z-projections of confocal sections. 1s, one-somite stage. See also Figure S5.
Conversely, heatshockactivation of dnBMPr mosaic embryos at oblong stage (dnBMPr-GFP expression is detectable from approximately dome stage onward; data not shown) most strikingly revealed that BMP-depleted cells inside the presump-tive telencephalon express the eye-field marker rx3 (Figures 5A–5H).This phenotype is extremely robust;however, the penetrance at the cellular level is slightly lower for dnBMPr mosaic (81%, Figure S5D) compared to caBMPr mosaic cells (92%, Figure S4D).The reason for this is likely due to residual BMP activity in a subset of dnBMPr cells. P-smad1,5,8 antibody staining indeed revealed that a low level of P-smad1,5,8 remains in approximately 40% of these transgenic cells (Figures S5A–S5C). The ectopic expression of rx3 can be interpreted as either (1) ectopic induction of rx3 inside the telencephalic dnBMPr-expressing cells, or (2) migration of rx3-positive eye-field cells into the telencephalon due to the loss of BMP activity. Two observations strongly support the first scenario. First, ectopic rx3 expression in BMP-depleted telencephalic cells has been detected at 75%-80% epiboly/midgastrula stage, coinciding with or very shortly after the on set of endogenous rx3 expression (Figures 5A and 5B). Second, we often find isolated single dnBMPr transgenic telencephalic cells at a distance from the eye field that ectopically express rx3 at 80% epiboly. These cells lack contact with the group of eye-field cells (arrows in Figures 5B and 5F), which indicates that the rx3 expression is due to ectopic induction rather than a migration.
Similar to caBMPr mosaics, ectopic induction of the eye-field marker rx3 did not occur when dnBMPr cells were heat shocked at shield stage (Figures 5M and 5N). These results support a temporal restriction in BMP requirement to the early phase of gastrulation and confirm that cells in the anterior neural plate (eye field/telencephalon) become insensitive to changes in level of BMP activity from midgastrula stage onward.
In addition to their ectopic expression of rx3 transcripts, dnBMPr-expressing telencephalic cells continue to express tlc, emx3, and foxg1 (Figures 5I–5L; data not shown), showing that these cells follow a dual-fate specification program. This observation contrasts with the Swrta72-/-- mutants in which anterior cells are all rx3 positive, and negative for telencephalic markers emx3 and foxg1 (Figures 1G, 1H, and 1M; data not shown).The ability to express these telencephalic markers is restored in BMP-depleted (DM-treated) rx3−/− embryos (Figures 2H–2J). This rescue demonstrates that Rx3 is the repressor of telencephalic fate in swr ta72−/−. The absence of repression of the telencephalic program in isolated clusters of rx3-positive cells strongly suggests that their telencephalic identity is maintained in the seclusters by the influence of the normal wild-type telencephalic surrounding. One strong possibility is that Rx3 is repressing telencephalon identity if expressed in all cells of key rostral signaling populations such as the ANB that normally imposes telencephalic fate.
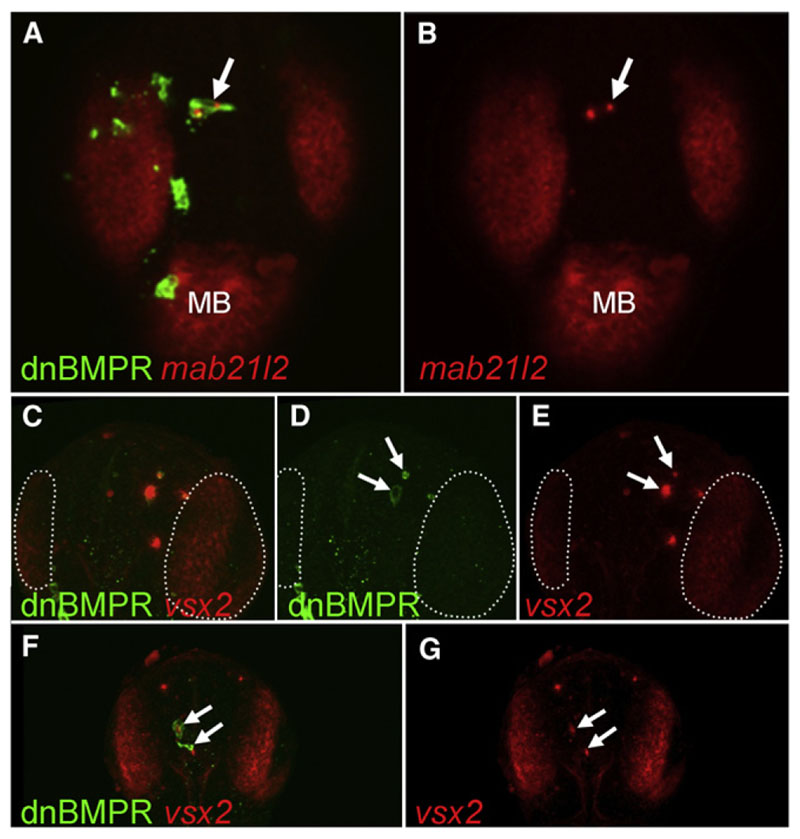
Telencephalic Cells Acquire Eye-like Fate in Response to BMP Depletion
In order to evaluate whether BMP-depleted cells located in a wild-type telencephalic environment are able to progress in their eye-like differentiation program, we analyzed retinal differ-entiation markers of eye development in the dnBMPr mosaics. Vsx2 is a specific marker of vertebrate retinal progenitor cells (Passini et al., 1997; Vitorino et al., 2009), and mab21l2 is expressed in the developing eye as well as in the midbrain and is required for eye morphogenesis (Kennedy et al., 2004; Rojas-Muñoz et al., 2005). At 24 hr postfertilization (hpf), the majority of transient transgenic cells no longer express dnBMPr-GFP. However, a few telencephalic cells are still GFP positive and express mab21l2 and vsx2 transcripts (Figure 6), showing that the ectopic activation of the eye differentiation program inside the telencephalon in response to BMP depletion is persistent rather than transient. The telencephalic markers foxg1 and emx3 were unaffected or slightly reduced in dnBMPr-GFP-positive cells at prim-5 staged (24 hpf) embryos, suggesting that these cells maintain a dual identity (Figure S6).
Figure 6. Ectopic Expression of Retinal Markers in BMP-Depleted Telencephalic Cells at 24 hpf.
(A-G) Embryos were injected with HSP70:dnBMPr plasmid at one- to two-cell stage followed by heat shock at oblong/early blastula stage. The arrows indicate dnBMPr-positive cells in the telencephalon at 24hr, which ectopically express retinal markers mab21l2 (A and B) and vsx2 (C-G). The number of transiently transgenic dnBMPr cells is significantly lower at 24 hpf than at earlier stages, and the remaining transgenic cells frequently undergo apoptosis (arrows in D). (C-E) White dotted lines mark the vsx2-positive retina. All views are dorsal, anterior at the top. All figures are z-projections of confocal sections. MB, midbrain. See also Figure S6.
The Chemokine Receptor cxcr4a Is Maintained by Rx3 in the Eye Field and Contributes to the Segregation of Eye and Telencephalic Cells during Neurulation
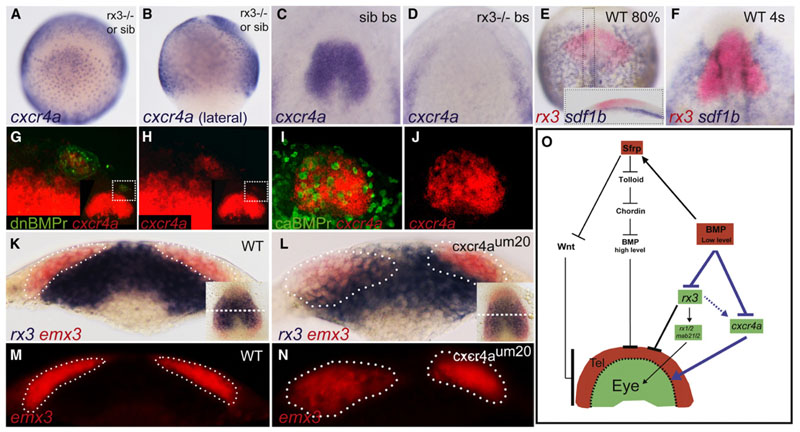
The very first distinction between eye-field and telencephalic precursors resides in their different morphogenetic behavior at the start of neurulation. From one to four somites, the telencephalic precursors first move on top of the eye field, toward the dorsal midline; then between five and eight somites, the eye field cells move outward to begin to form the eye vesicles. One major role of P-Smad1,5,8 inside the rostral neural plate of early gastrula stage embryos may therefore be to protect the telencephalic precursors from mixing with the eye cells when initiating their medial migration at early neurulation stage. We therefore tested several potential downstream targets of both the BMP pathway and Rx3 that may be involved in conferring specific motility or differential adhesive properties to the telencephalon and eye field, respectively. A strong candidate is the chemokine receptor cxcr4a, which has been shown to control cell movements as well as adhesion in many contexts (Engl et al., 2006; Hartmann et al., 2005; Miyasaka et al., 2007; Mizoguchi et al., 2008; Nair and Schilling, 2008). Indeed, cxcr4a is strongly expressed in the eye field at bud stage, whereas the corresponding ligands sdf1a/b are expressed in the mesendoderm underlying the eye field (Figures 7C, 7E, and 7F; Mizoguchi et al., 2008). Cxcr4a eye-field expression is initially induced in rx3−/− mutant embryos at lower levels (75% epiboly/midgastrula stage) but is then completely lost by the end of gastrulation, whereas its transcription is maintained in the lateral nonneural tissue (Figures 7A–7D; data not shown). Sdf-1b expression remains unchanged in rx3−/− embryos (data not shown). Identical to rx3, cxcr4a expression is ectopically induced in dnBMPr transgenic cells inside the telencephalon (Figures 7G, 7H, and S5) and repressed in caBMPr transgenic cells (Figures 7I, 7J, and S4). Thus, whereas Rx3 is required to maintain cxcr4a expression in the eye field, BMP acts as a cxcr4a repressor in the telencephalon (Figure 7O).
Figure 7. The Chemokine Receptor cxcr4a Demarcates the Border of the Eye Field and Telencephalon Downstream of BMP and Rx3.
(A-D) Cxcr4a expression in rx3−/− or sib at 80% epiboly (A and B) and at bud stage in sib (C) and rx3−/− (D) embryos. Sdf1b/rx3 double in situ at 80% epiboly/midgastrula stage (E) and four-somite stage (4s) (F). Inset in (E) shows a sagittal section of the boxed area,anteriorto the left.
(G-J) Cxcr4a transcript expression (red) in dnBMPr transgenic mosaics (green in G and H) and caBMPr transgenic mosaics (green in I and J) at bud stage. (G and H) Close-up of the boxed area marked in the inset. (I and J) Repression of cxcr4a expression in caBMPr-overexpressing cells.
(K-N) Cross-section at med ial A-P level of the eye field (dashed linein the insets in K and L). Segregation ofrx3 (blue) and emx3(red) expression domainsin WT (K and M) and cxcr4aum20+/- x cxcr4aum20+/- crosses (Land N; n = 14/61) at four-somite stage. Dotted lines mark the border of emx3-expressing cells as defined by fluorescent images (M and N).
(O) A model of the genetic network leading to formation of the eye and telencephalon. Blue arrows demonstrate the present work.Dotted-arrow line indicates that Rx3 is not essential for induction but for maintenance of cxcr4a expression.
See also Figure S7.
To access whether Cxcr4a plays a role in morphogenetic movements of eye field and telencephalon downstream of Rx3 and BMP signaling, we analyzed rx3/emx3 expression at bud and early somite stages in the cxcr4a mutant line. This line carries a genomic lesion leading to the generation of a Crxr4a protein, which is unable to localize in the membrane (Siekmann et al., 2009). Although whole mounts did not show any irregular expression pattern (inset in Figures 7K and 7L), in cross-sections approximately 25% of the progeny of heterozygous crosses (Mendelian proportion of homozygous mutant embryos) clearly show intermingling of cells between emx3 and rx3-expressing cells at four- to five-somite stage (Figures 7L and 7N; n = 14/61). In contrast, sectioning of bud stage embryos did not reveal any abnormalities (data not shown). These results indicate that Cxcr4a confers specific segregative properties to the eye field, which is required for correct neurulation, as soon as telencephalic cells start to move on top of the eye precursors during the early stage of neurulation.
Discussion
During midgastrulation, repression of the Wnt/b-catenin pathway, through secretion of pathway antagonists from the ANB, is a major requirement for establishment of the telencephalon (Houart et al., 1998, 2002; Niehrs, 1999). Our present study reveals that a specific level of BMP activity is required at even earlier stages (blastula to early gastrula) to establish telencephalic identity by preventing eye identity along the ANB. In the absence of BMP activity, the key eye inducer and potent telencephalon repressor, the transcription factor Rx3, is expressed in the prospective telencephalon. As a result telencephalic precursors express eye-specific transcripts and are able to pursue a retinal differentiation program. Complementarily, repression of BMP activity inside the anterior forebrain anlage is necessary for rx3 expression and eye differentiation. The BMP-driven mechanism that we describe here has a major impact on the type of movement telencephalon and eye precursors will undertake during neurulation. We demonstrate that the chemokine receptor cxcr4a is acting downstream of Rx3 to maintain an essential physical border between eye-field cells and medially converging telencephalic cells during early neurulation.
The Spatial-Temporal Activity of the BMP Pathway Is Dynamic during Zebrafish Gastrulation
In zebrafish, BMP patterns the D-V axis in a temporarily progressive manner along the A-P axis (Tucker et al., 2008). The BMP inhibition results presented here support such temporal progres-sion and extend it to A/P patterning in the forebrain because cells at the anteriormost tip of the neural plate (future telencephalic subpallium) only respond to changes in BMP activity prior to 60% epiboly/early gastrula stage and become unresponsive to depletion of BMP signaling thereafter. Furthermore, heat shock of dnBMPr and caBMPr at dome stage but not at shield stage causes changes in neural plate fates. In addition to the temporal change in BMP requirement, the activity of the BMP pathway is also temporally controlled inside the neural territory, as shown by the dynamic changes of the P-Smad1,5,8 and BRE-GFP gradient during gastrulation. The dynamics of Psmad 1,5,8 distribution during gastrulation reflects the previously described two-phase model (early BMP requirement at blastula and full BMP attenuation at late gastrula stages) for the establishment of preplacodal competence in zebrafish (Kwon et al., 2010).
Eye-Field Markers Are Repressed by BMP Activity in the Telencephalon Anlage
We have found several putative Smad binding elements (SBEs) in the basal promoter region of the rx3 genomic sequence (data not shown). However, the time window of both responsiveness to BMP (until midgastrula)and onset of rx3 expression (from midgastrula onward) is only slightly overlapping. Thus, it seems unlikely that BMP represses rx3 in a direct fashion. A microarray-based, ANB-specific transcriptome will be screened to identify early transcription factors that are sensitive to BMP attenuation and able to repress rx3 expression. Also, we did not find any evidence for a mutual inhibition between BMP and Rx3. The distribution of P-smad1,5,8 in rx3−/− mutant embryos at late gastrula or bud stage was unaffected (data not shown). Furthermore, we did not detect any upregulation of BMP down-stream targets in a microarray analysis that we performed between wild-type and rx3−/− embryos (unpublished data).
Analysis of BMP signaling in chick embryos suggests the paraxial mesoderm to be involved in shaping eye invagination during neurulation because loss of mesodermal BMP signal leads to increase of optical vesicle size (Teraoka et al., 2009). BMP may therefore regulate the formation of the eye vesicle throughout its development, from gastrulation to end of neurulation.
The Telencephalon Is Established by Specific Levels of BMP Signaling Activity
The mosaic overexpression of caBMPr results in high levels of BMP activity, which causes repression of foxg1 in a non-cell-autonomous manner. However, costaining with P-Smad1,5,8 antibody showed that caBMPr induces the BMP pathway in a cell-autonomous manner. This could indicate the presence of a yet-unknown secreted factor downstream of BMP, expressed by the caBMPr-positive cells and able to repress the telencephalic marker foxg1 in neighboring cells. Conversely, it is conceivable that a protelencephalic factor is maintaining telencephalic fate in wild-type embryos; however, it is repressed by caBMPr cells, leading to loss of telencephalic markers in neighboring cells. This could as well explain the mixed fate (eye, telencephalon) of mosaic dnBMPr cells because they still receive the protelencephalic signal from their neighboring wildtype cells in contrast to swr mutants.
Our caBMPr overexpression data confirm the neural default model (Hemmati-Brivanlou and Melton, 1997; Muñoz-Sanjuàn and Brivanlou, 2002; Weinstein and Hemmati-Brivanlou, 1997), showing that a very high level of BMP activity represses neural fate. However, our overall results require a further refinement of the model both temporarily and spatially in order to account for the requirement of BMP in the anterior neural tissue as early as late blastula stage (Figure S7). Studies in frog may seem to contrast with these results because it has been shown that continued suppression of BMP throughout gastrula stages is required for neural specification (Hartley et al., 2001; Khokha et al., 2005). However, this apparent divergence is due to the use of broad rostral markers and presence of a very high level of BMP in experimental embryos in these studies.
In mouse, P-Smad1,5,8 antibody staining also indicates that BMP activity briefly overlaps with the expression of anterior neural markers (six3/hesx1) at the anterior-proximal margin (Yang and Klingensmith, 2006). Therefore, at early stages of neural plate development, BMP activity and prospective forebrain fates are overlapping in fish and mammals, suggesting that the requirement of BMP activity for establishment of telen-cephalic fate is conserved throughout vertebrates.
Downstream of BMP: How Does Cxcr4 Influence the Differential Migration of Eye-Field versus Telencephalon Cells?
An early distinction of fate between telencephalon and eye precursors inside the neural plate is of key importance because it is a prerequisite to the differential morphogenetic movements leading to the dorsal convergence of telencephalic cells and formation of the eye vesicle during neurulation (Martinez-Morales and Wittbrodt, 2009). Can BMP be involved in the segregation of these two groups of cells? Here we show that the chemokine receptor cxr4a is expressed in the eye field, under the control of Rx3(atbudstage), and absent in thetelencephalon where it is repressed by BMP. Interestingly, Rx3alone proved to be insufficient to induce cxcr4a expression when mis-expressed in the telencephalon (data not shown).This fits to our observation that in Rx3−/− mutant embryos cxcr4a expression is induced, but not maintained, suggesting the requirement of a Rx3 cofactor located in the eye field.Besides its role in cell migration in zebrafish (Mizoguchi et al.,2008; Nair and Schilling, 2008), it has been shown that activation of the Cxcr4 receptor leads to an integrinmediated increased adhesion in human cancer cells and during zebrafish gastrulation (Engl et al., 2006; Hartmann et al., 2005; Nair and Schilling, 2008). Migration of cxcr4a-expressing cells (ectoderm) toward the ligands (endoderm) can be ruled out because no vertical cell migration occurs in the eye field. However, before the eye vesicles evaginate, the eye-field cells necessitate a firm adhesion to remain as a compact group and to provide a cohesive surface for telencephalic cells that undergo lateral-to-medial convergence on top of the eye field. Thus, loss of Cxcr4a function may result in a decrease of adhesion in the eye field, thereby allowing telencephalic cells to leave their normal morphogenetic path and intermingle with eye-field cells as shown in cxcr4aum20−/− embryos. In future experiments we aim to investigate differential adhesive properties of both cell groups and perform time-lapse imaging of the cxcr4aum20 3 Rx3-GFP line to analyze the malformed morphogenesis in cxcr4a mutant embryos in more detail.
BMP and the Telencephalic Dorsal Signaling Center
Besides its role in early ectoderm patterning, BMP, together with Shh and Wnts, is also known to be the crucial signal in establishing the dorsal ventral axis in the spinal cord and the forebrain (Furuta et al., 1997; Tanabe and Jessell, 1996). There is compelling evidence that BMP activity from the roof plate patterns the dorsal telencephalon (Cheng et al., 2006; Monuki et al., 2001; Shimogori et al., 2004; Theil et al., 2002). The set of data presented here shows that BMP signaling is activated within the presumptive nascent telencephalon (including both perspective pallium and subpallium fates) during early gastrulation. We propose that the influence of BMP activity confers a uniform dorsal identity to the developing telencephalon, which is progressively repressed at the onset of Shh activity, driving subpallial fate. This hypothesis, although contradicting chick explant experiments(Gunhaga et al.,2000,2003),is compatible with the complex Foxg1-controlled regulatory network we uncovered recently (Danesin et al., 2009).
Experimental Procedures
Zebrafish Lines
All of the zebrafish studies were performed with approval from the UK Home Office under a HO project license to C.H. Lines used were swrta72 (bmp2b−/−) (Kishimoto et al., 1997), dino tt250) (chordin −/−) (Schulte-Merker et al., 1997), (rx3 −/−) (Loosli et al., 2003), BRE:GFP (Alexander et al., 2011), and cxcr4aum20 (Siekmann et al., 2009). Fish were maintained on a 14 hr light/1 0 hr dark cycle in fish facilities at King’s College.
DM Treatment
A DM (Calbiochem; 1 71 260) stock solution was prepared at a concentration of 5 μM in DMSO. Prior to DM treatment embryos were transferred to 4-well plates, and the chorion was opened. Embryos were incubated in DM at times and concentrations as indicated in Figure 2. Control embryos were incubated in fish watercontaining an equal concentration of DMSO to that of DM-treated embryos.
Generation of Transiently Transgenic Zebrafish Embryos
For local gain and loss of BMP function, heat-shock-inducible caBMPrI (HA tagged; Quillien et al., 2011) and dnBMPrI (C-terminal GFP fusion; Pyati et al., 2005) constructs were injected at one- and two-cell stage together with tol2 and meganuclease Isce-I, respectively. Heat shock (37°C for 30 min) was performed at times indicated, resulting in a mosaic overexpression of dnBMPr-GFP from 1 hr after heat shock. After heat shock, embryos were kept at 28°C and fixed with 4% PFA at times indicated.
Whole-Mount In Situ Hybridization and Immunohistochemistry
Standard procedures were followed for in situ hybridization analysis using full-length probes of foxg1, rx3, emx3, dlx3, mab21l2, vsx2 (kindly provided by the Harris lab, University of Cambridge UK), cxcr4a (kindly provided by Dr. R. Knight), sdf1b (kindly provided by the Raz lab, Munster, Germany), and her5. For “Fast Red” in situ staining, embryos were incubated in Fast Red (Roche) substrate solution (0.1 M Tris-HCl, 100 μM NaCl [pH 8.2]) for 2 or more hours at RT (or at 4°C overnight). For immunohistochemistry the following antibodies were used: anti-GFP (Torrey Pines Biolabs) for detection of dnBMPr-GFP; anti-HA (Sigma-Aldrich) for detection of HA-tagged caBMPr; and anti-P-Smad1,5,8 (Cell Signaling Technology). Embryos were fixed in 4% PFA at 4°C overnight, blocked in goat serum-PBST (10% goat serum, 0.1% Tween 20 in PBS; 1% DMSO added for P-Smad1/5/8 antibody samples), and incubated overnight in a dilution of primary antibody (anti-GFP 1:500, anti-HA 1:500, anti-P-Smad1,5,8 1:100) in PBT/1% goat serum. PBT washes (3310 min) were followed by a 1 :500 dilution of secondary antibody coupled to Alexa Fluor 488/568 (Invitrogen). Confocal imaging was done by using a Nikon Eclipse C1 microscope. Images were processed using ImageJ and Adobe software.
RNA/Morpholino Injections
Capped dkk1 RNA was transcribed with SP6 RNA polymerase using the mMESSAGE mMACHINE Kit (Ambion) and injected at one-cell stage at 50 ng/ml. Dkk1 morpholino antisense oligonucleotides (GeneTools) were used at a concentration of 2 ng/nl.
Supplementary Material
Supplemental Information includes seven figures and can be found with this article online at http://dx.doi.org/10.1016/j.devcel.2012.09.006.
Acknowledgments
We thank David Kimelman for the HSP70:dnBmpr-GFP construct and Patrick Blader for the HSP70:HA-caBMPr construct. We would also like to thank Arndt Siekmann for sending us fixed cxcr4aum20+/- X cxcr4aum20+/- embryos and Ken Cho and Tom Schilling for the BRE:GFP zebrafish line, respectively. We are grateful to Andrea Streit, Klaus Michael Kuerner, and Pari Mueller for helpful comments on the manuscript. H.B. was funded by the German Research Foundation (DFG). This work was funded by the Medical Research Council, EU, FP7 ZF-HEALTH and the Wellcome Trust awarded to C.H.
References
- Alexander C, Zuniga E, Blitz IL, Wada N, Le Pabic P, Javidan Y, Zhang T, Cho KW, Crump JG, Schilling TF. Combinatorial roles for BMPs and Endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton. Development. 2011;138:5135–5146. doi: 10.1242/dev.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AP, Streit A. Sensory organs: making and breaking the pre-placodal region. Curr Top Dev Biol. 2006;72:167–204. doi: 10.1016/S0070-2153(05)72003-2. [DOI] [PubMed] [Google Scholar]
- Barth KA, Kishimoto Y, Rohr KB, Seydler C, Schulte-Merker S, Wilson SW. Bmp activity establishes a gradient of positional information throughout the entire neural plate. Development. 1999;126:4977–4987. doi: 10.1242/dev.126.22.4977. [DOI] [PubMed] [Google Scholar]
- Cheng X, Hsu CM, Currle DS, Hu JS, Barkovich AJ, Monuki ES. Central roles of the roof plate in telencephalic development and holo-prosencephaly. J Neurosci. 2006;26:7640–7649. doi: 10.1523/JNEUROSCI.0714-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesin C, Peres JN, Johansson M, Snowden V, Cording A, Papalopulu N, Houart C. Integration of telencephalic Wnt and hedgehog signaling center activities by Foxg1. Dev Cell. 2009;16:576–587. doi: 10.1016/j.devcel.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Delaune E, Lemaire P, Kodjabachian L. Neural induction in Xenopus requires early FGF signalling in addition to BMP inhibition. Development. 2005;132:299–310. doi: 10.1242/dev.01582. [DOI] [PubMed] [Google Scholar]
- Dick A, Meier A, Hammerschmidt M. Smad1 and Smad5 have distinct rolesduring dorsoventral patterning ofthezebrafish embryo. Dev Dyn. 1999;216:285–298. doi: 10.1002/(SICI)1097-0177(199911)216:3<285::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Dutta S, Dietrich JE, Aspöck G, Burdine RD, Schier A, Westerfield M, Varga ZM. pitx3 defines an equivalence domain for lens and anterior pituitary placode. Development. 2005;132:1579–1590. doi: 10.1242/dev.01723. [DOI] [PubMed] [Google Scholar]
- Engl T, Relja B, Marian D, Blumenberg C, Müller I, Beecken WD, Jones J, Ringel EM, Bereiter-Hahn J, Jonas D, Blaheta RA. CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia. 2006;8:290–301. doi: 10.1593/neo.05694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y, Piston DW, Hogan BL. Bone morphogenetic proteins (BMPs) as regulators of dorsal forebrain development. Development. 1997;124:2203–2212. doi: 10.1242/dev.124.11.2203. [DOI] [PubMed] [Google Scholar]
- Glavic A, Honoré S Maris, Feijóo C Gloria, Bastidas F, Allende ML, Mayor R. Role of BMP signaling and the homeoprotein Iroquois in the specification of the cranial placodal field. Dev Biol. 2004;272:89–103. doi: 10.1016/j.ydbio.2004.04.020. [DOI] [PubMed] [Google Scholar]
- Gunhaga L, Jessell TM, Edlund T. Sonic hedgehog signaling at gastrula stages specifies ventral telencephalic cells in the chick embryo. Development. 2000;127:3283–3293. doi: 10.1242/dev.127.15.3283. [DOI] [PubMed] [Google Scholar]
- Gunhaga L, Marklund M, Sjödal M, Hsieh JC, Jessell TM, Edlund T. Specification of dorsal telencephalic character by sequential Wnt and FGF signaling. Nat Neurosci. 2003;6:701–707. doi: 10.1038/nn1068. [DOI] [PubMed] [Google Scholar]
- Hammerschmidt M, Pelegri F, Mullins MC, Kane DA, van Eeden FJ, Granato M, Brand M, Furutani-Seiki M, Haffter P, Heisenberg CP, et al. dino and mercedes, two genes regulating dorsal development in the zebrafish embryo. Development. 1996;123:95–102. doi: 10.1242/dev.123.1.95. [DOI] [PubMed] [Google Scholar]
- Hartley KO, Hardcastle Z, Friday RV, Amaya E, Papalopulu N. Transgenic Xenopus embryos reveal that anterior neural development requires continued suppression of BMP signaling after gastrulation. Dev Biol. 2001;238:168–184. doi: 10.1006/dbio.2001.0398. [DOI] [PubMed] [Google Scholar]
- Hartmann TN, Burger JA, Glodek A, Fujii N, Burger M. CXCR4 chemokine receptor and integrin signaling co-operate in mediating adhesion and chemoresistance in small cell lung cancer (SCLC) cells. Oncogene. 2005;24:4462–4471. doi: 10.1038/sj.onc.1208621. [DOI] [PubMed] [Google Scholar]
- Heisenberg CP, Houart C, Take-Uchi M, Rauch GJ, Young N, Coutinho P, Masai I, Caneparo L, Concha ML, Geisler R, et al. A mutation in the Gsk3-binding domain of zebrafish Masterblind/Axin1 leads to a fate transformation of telencephalon and eyes to diencephalon. Genes Dev. 2001;15:1427–1434. doi: 10.1101/gad.194301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati-Brivanlou A, Melton D. Vertebrate neural induction. Annu Rev Neurosci. 1997;20:43–60. doi: 10.1146/annurev.neuro.20.1.43. [DOI] [PubMed] [Google Scholar]
- Houart C, Westerfield M, Wilson SW. A small population of anterior cells patterns the forebrain during zebrafish gastrulation. Nature. 1998;301:788–792. doi: 10.1038/35853. [DOI] [PubMed] [Google Scholar]
- Houart C, Caneparo L, Heisenberg C, Barth K, Take-Uchi M, Wilson S. Establishment of the telencephalon during gastrulation by local antagonism of Wnt signaling. Neuron. 2002;35:255–265. doi: 10.1016/s0896-6273(02)00751-1. [DOI] [PubMed] [Google Scholar]
- Kennedy BN, Stearns GW, Smyth VA, Ramamurthy V, van Eeden F, Ankoudinova I, Raible D, Hurley JB, Brockerhoff SE. Zebrafish rx3 and mab21l2 are required during eye morphogenesis. Dev Biol. 2004;270:336–349. doi: 10.1016/j.ydbio.2004.02.026. [DOI] [PubMed] [Google Scholar]
- Khokha MK, Yeh J, Grammer TC, Harland RM. Depletion of three BMP antagonists from Spemann’s organizer leads to a catastrophic loss of dorsal structures. Dev Cell. 2005;8:401–411. doi: 10.1016/j.devcel.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Kiecker C, Niehrs C. A morphogen gradient of Wnt/beta-catenin signalling regulates anteroposterior neural patterning in Xenopus. Development. 2001;128:4189–4201. doi: 10.1242/dev.128.21.4189. [DOI] [PubMed] [Google Scholar]
- Kishimoto Y, Lee KH, Zon L, Hammerschmidt M, Schulte-Merker S. The molecular nature of zebrafish swirl: BMP2 function is essential during early dorsoventral patterning. Development. 1997;124:4457–4466. doi: 10.1242/dev.124.22.4457. [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW, Rossi CC, Artinger KB. Mechanisms driving neural crest induction and migration in the zebrafish and Xenopus laevis. Cell Adhes Migr. 2010;4:595–608. doi: 10.4161/cam.4.4.12962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HJ, Bhat N, Sweet EM, Cornell RA, Riley BB. Identification of early requirements for preplacodal ectoderm and sensory organ development. PLoS Genet. 2010;6:e1001133. doi: 10.1371/journal.pgen.1001133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon YG, Mullins MC. Maternal and zygotic control of zebrafish dorsoventral axial patterning. Annu Rev Genet. 2011;45:357–377. doi: 10.1146/annurev-genet-110410-132517. [DOI] [PubMed] [Google Scholar]
- Linker C, Stern CD. Neural induction requires BMP inhibition only as a late step, and involves signals other than FGF and Wnt antagonists. Development. 2004;131:5671–5681. doi: 10.1242/dev.01445. [DOI] [PubMed] [Google Scholar]
- Loosli F, Staub W, Finger-Baier KC, Ober EA, Verkade H, Wittbrodt J, Baier H. Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO Rep. 2003;4:894–899. doi: 10.1038/sj.embor.embor919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morales JR, Wittbrodt J. Shaping the vertebrate eye. Curr Opin Genet Dev. 2009;19:511–517. doi: 10.1016/j.gde.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Mathers PH, Grinberg A, Mahon KA, Jamrich M. The Rx homeobox gene is essential for vertebrate eye development. Nature. 1997;387:603–607. doi: 10.1038/42475. [DOI] [PubMed] [Google Scholar]
- Miyasaka N, Knaut H, Yoshihara Y. Cxcl12/Cxcr4 chemokine signaling is required for placode assembly and sensory axon pathfinding in the zebrafish olfactory system. Development. 2007;134:2459–2468. doi: 10.1242/dev.001958. [DOI] [PubMed] [Google Scholar]
- Mizoguchi T, Verkade H, Heath JK, Kuroiwa A, Kikuchi Y. Sdf1/Cxcr4 signaling controlsthe dorsal migration ofendodermal cells during zebrafish gastrulation. Development. 2008;135:2521–2529. doi: 10.1242/dev.020107. [DOI] [PubMed] [Google Scholar]
- Monuki ES, Porter FD, Walsh CA. Patterning of the dorsal telencephalon and cerebral cortex by a roof plate-Lhx2 pathway. Neuron. 2001;32:591–604. doi: 10.1016/s0896-6273(01)00504-9. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay M, Shtrom S, Rodriguez-Esteban C, Chen L, Tsukui T, Gomer L, Dorward DW, Glinka A, Grinberg A, Huang SP, et al. Dickkopf1 is required for embryonic head induction and limb morphogenesis in the mouse. Dev Cell. 2001;1:423–434. doi: 10.1016/s1534-5807(01)00041-7. [DOI] [PubMed] [Google Scholar]
- Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, et al. Genes establishing dorsoventral pattern formation inthezebrafish embryo: the ventral specifying genes. Development. 1996;123:81–93. doi: 10.1242/dev.123.1.81. [DOI] [PubMed] [Google Scholar]
- Muñoz-Sanjuàn I, Brivanlou AH. Neural induction, the default model and embryonic stem cells. Nat Rev Neurosci. 2002;3:271–280. doi: 10.1038/nrn786. [DOI] [PubMed] [Google Scholar]
- Nair S, Schilling TF. Chemokine signaling controls endodermal migration during zebrafish gastrulation. Science. 2008;322:89–92. doi: 10.1126/science.1160038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VH, Schmid B, Trout J, Connors SA, Ekker M, Mullins MC. Ventral and lateral regions of the zebrafish gastrula, including the neural crest progenitors, are established by a bmp2b/swirl pathway of genes. Dev Biol. 1998;199:93–110. doi: 10.1006/dbio.1998.8927. [DOI] [PubMed] [Google Scholar]
- Nguyen VH, Trout J, Connors SA, Andermann P, Weinberg E, Mullins MC. Dorsal and intermediate neuronal cell types of the spinal cord are established by a BMP signaling pathway. Development. 2000;127:1209–1220. doi: 10.1242/dev.127.6.1209. [DOI] [PubMed] [Google Scholar]
- Niehrs C. Head in the WNT: the molecular nature of Spemann’s head organizer. Trends Genet. 1999;15:314–319. doi: 10.1016/s0168-9525(99)01767-9. [DOI] [PubMed] [Google Scholar]
- Nordström U, Jessell TM, Edlund T. Progressive induction of caudal neural character by graded Wnt signaling. Nat Neurosci. 2002;5:525–532. doi: 10.1038/nn0602-854. [DOI] [PubMed] [Google Scholar]
- Passini MA, Levine EM, Canger AK, Raymond PA, Schechter N. Vsx-1 and Vsx-2: differential expression of two paired-like homeobox genes during zebrafish and goldfish retinogenesis. J Comp Neurol. 1997;388:495–505. doi: 10.1002/(sici)1096-9861(19971124)388:3<495::aid-cne11>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Patthey C, Gunhaga L. Specification and regionalisation of the neural plate border. Eur J Neurosci. 2011;34:1516–1528. doi: 10.1111/j.1460-9568.2011.07871.x. [DOI] [PubMed] [Google Scholar]
- Pera E, Kessel M. Expression of DLX3 in chick embryos. Mech Dev. 1999;89:189–193. doi: 10.1016/s0925-4773(99)00207-5. [DOI] [PubMed] [Google Scholar]
- Pyati UJ, Webb AE, Kimelman D. Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development. 2005;132:2333–2343. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- Quillien A, Blanco-Sanchez B, Halluin C, Moore JC, Lawson ND, Blader P, Cau E. BMP signaling orchestrates photoreceptor specification in the zebrafish pineal gland in collaboration with Notch. Development. 2011;138:2293–2302. doi: 10.1242/dev.060988. [DOI] [PubMed] [Google Scholar]
- Rojas-Muñoz A, Dahm R, Nüsslein-Volhard C. chokh/rx3 specifies the retinal pigment epithelium fate independently of eye morphogenesis. Dev Biol. 2005;288:348–362. doi: 10.1016/j.ydbio.2005.08.046. [DOI] [PubMed] [Google Scholar]
- Sasai Y, De Robertis EM. Ectodermal patterning in vertebrate embryos. Dev Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Awtry T, Brugmann SA, Jensen ED, Neilson K, Ruan G, Stammler A, Voelker D, Yan B, Zhang C, et al. Eya1 and Six1 promote neurogenesis in the cranial placodes in a SoxB1-dependent fashion. Dev Biol. 2008;320:199–214. doi: 10.1016/j.ydbio.2008.05.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Schumacher JA, Hashiguchi M, Nguyen VH, Mullins MC. An intermediate level of BMP signaling directly specifies cranial neural crest progenitor cells in zebrafish. PLoS One. 2011;6:e27403. doi: 10.1371/journal.pone.0027403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimogori T, Banuchi V, Ng HY, Strauss JB, Grove EA. Embryonic signaling centers expressing BMP, WNT and FGF proteins interact to pattern the cerebral cortex. Development. 2004;131:5639–5647. doi: 10.1242/dev.01428. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Standley C, Fogarty KE, Wolfe SA, Lawson ND. Chemokine signaling guides regional patterning of the first embryonic artery. Genes Dev. 2009;23:2272–2277. doi: 10.1101/gad.1813509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigloher C, Ninkovic J, Laplante M, Geling A, Tannhäuser B, Topp S, Kikuta H, Becker TS, Houart C, Bally-Cuif L. Segregation of telencephalic and eye-field identities inside the zebrafish forebrain territory is controlled by Rx3. Development. 2006;133:2925–2935. doi: 10.1242/dev.02450. [DOI] [PubMed] [Google Scholar]
- Streit A, Berliner AJ, Papanayotou C, Sirulnik A, Stern CD. Initiation of neural induction by FGF signalling before gastrulation. Nature. 2000;406:74–78. doi: 10.1038/35017617. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- Teraoka ME, Paschaki M, Muta Y, Ladher RK. Rostral paraxial mesoderm regulates refinement of the eye field through the bone morphogenetic protein (BMP) pathway. Dev Biol. 2009;330:389–398. doi: 10.1016/j.ydbio.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Theil T, Aydin S, Koch S, Grotewold L, Rüther U. Wnt and Bmp signalling cooperatively regulate graded Emx2 expression in the dorsal telencephalon. Development. 2002;129:3045–3054. doi: 10.1242/dev.129.13.3045. [DOI] [PubMed] [Google Scholar]
- Tribulo C, Aybar MJ, Nguyen VH, Mullins MC, Mayor R. Regulation of Msx genes by a Bmp gradient is essential for neural crest specification. Development. 2003;130:6441–6452. doi: 10.1242/dev.00878. [DOI] [PubMed] [Google Scholar]
- Tucker JA, Mintzer KA, Mullins MC. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev Cell. 2008;14:108–119. doi: 10.1016/j.devcel.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitorino M, Jusuf PR, Maurus D, Kimura Y, Higashijima S, Harris WA. Vsx2 in the zebrafish retina: restricted lineages through derepression. Neural Dev. 2009;4:14. doi: 10.1186/1749-8104-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein DC, Hemmati-Brivanlou A. Neural induction in Xenopus laevis: evidence for the default model. Curr Opin Neurobiol. 1997;7:7–12. doi: 10.1016/s0959-4388(97)80114-6. [DOI] [PubMed] [Google Scholar]
- Wilson PA, Hemmati-Brivanlou A. Vertebrate neural induction: inducers, inhibitors, and a new synthesis. Neuron. 1997;18:699–710. doi: 10.1016/s0896-6273(00)80311-6. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Rubenstein JL. Induction and dorsoventral patterning of the telencephalon. Neuron. 2000;28:641–651. doi: 10.1016/s0896-6273(00)00171-9. [DOI] [PubMed] [Google Scholar]
- Wilson SW, Houart C. Early steps in the development of the forebrain. Dev Cell. 2004;6:167–181. doi: 10.1016/s1534-5807(04)00027-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang YP, Klingensmith J. Roles of organizer factors and BMP antagonism in mammalian forebrain establishment. Dev Biol. 2006;296:458–475. doi: 10.1016/j.ydbio.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.