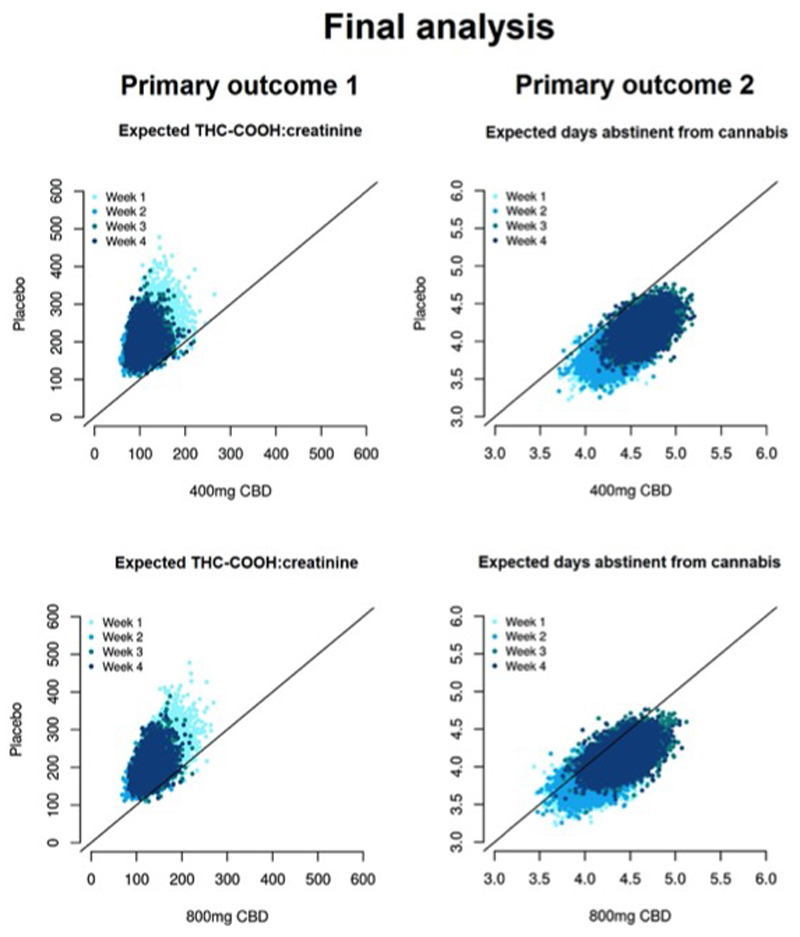
Figure 2.
Primary endpoint: final analysis. Each cloud of points represents the expected value of the primary outcome for participants randomised to CBD or placebo, simulated from the joint posterior distribution of treatment arms. Both 400mg and 800mg CBD exceed the upper threshold (Pr=0.9) for primary endpoint criteria, showing reduced THC-COOH:creatinine concentrations (left panel) and increased days per week abstinent from cannabis (right panel) compared to placebo.