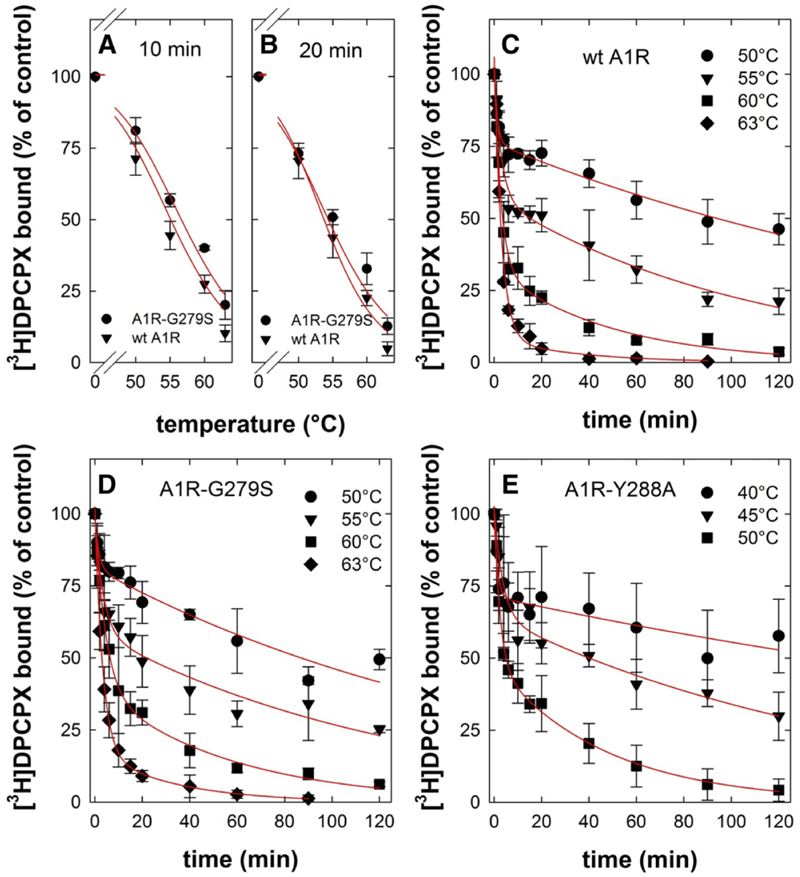
Fig. 3.
Heat-induced denaturation of wild-type and mutant adenosine A1-receptors. (A and B) Membranes were prepared from HEK293 cells transiently expressing the FLAG-tagged human wildtype (wt A1R, closed triangles) and mutant A1-receptor (A1R-G279S7.44, closed circles). Membranes (3–5 μg/assay) were subjected to heat-induced denaturation and incubated for 10 minutes (A) or 20 minutes (B) at the indicated temperatures. Thereafter, the membranes were placed on ice. Binding of the [3H]DPCPX (3 nM) was determined for 1 hour at 0°C. The curves in (A and B) were drawn by fitting the data to a three-parameter logistic equation. (C–E) Membranes harboring FLAG-tagged A1R-Y288A7.53 (closed squares) were prepared from transiently transfected HEK293 cells, which had been incubated in the presence of 100 μM 3-isobutyl-1-methylxanthine to restore folding and surface expression of the receptor prior to cell lysis. Membranes (3–5 μg/assay for wt A1R and A1R-G279S7.44; 10–15 μg/assay for A1R-Y288A7.53) were subjected to de-naturation at the indicated temperatures and for the indicated time intervals. Thereafter, binding of [3H]DPCPX (3 nM) was determined as outlined for (A and B). Nonspecific binding was defined in the presence of 10 μM XAC; it was <10% of total binding, did not change with temperature or time, and was subtracted. The 100% reference value is binding to control membranes, which were held on ice throughout the experiment. This binding was 10–15 fmol/assay. Data are means ± S.D. from three independent experiments, which were done in parallel. The curves in (C–E) were drawn by fitting the data to the equation for a biexponential decay.