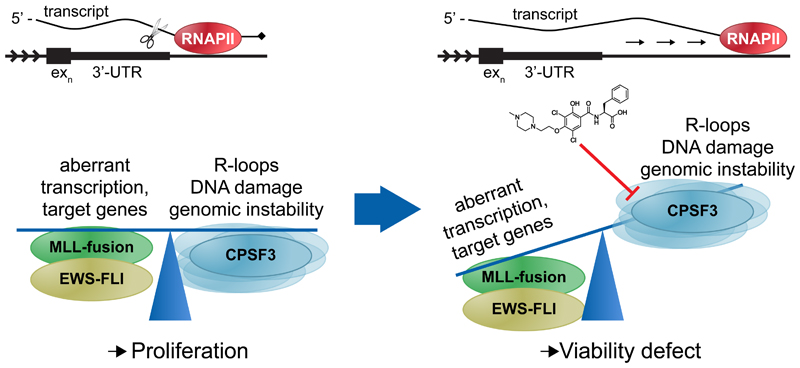
Figure 6. Proposed model for compound 2 mechanism of action.
Cancers such as EWSFLI translocated Ewing’s sarcoma or MLL-translocated AML have selected for a balance between oncogenic fusion protein-driven aberrant transcription and its associated genomic instability that allows for proliferation. Under such conditions, the mRNA processing and termination machinery, specifically one of its core components, the cleavage and polyadenylation specificity factor 3 (CPSF3), represents a previously undescribed novel synthetic lethal node. Compound 2 inhibition of CPSF3-mediated mRNA cleavage results in RNA Pol II read-through beyond the 3’-UTR and transcript accumulation. As a result, gene expression is perturbed, including downregulation of genes involved in DNA double-strand break repair, while R-loop formation is increased to levels that the cell can no longer buffer or balance, ultimately leading to a cell viability defect.