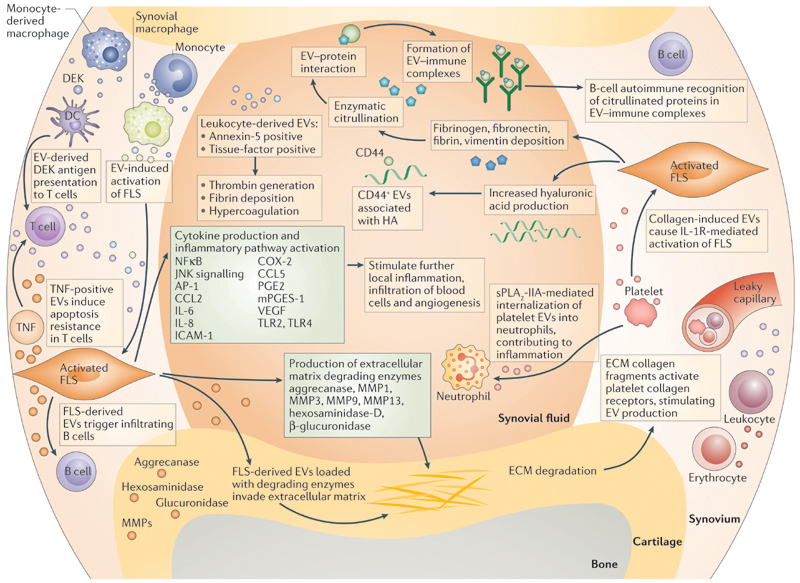
Figure 1. Proposed mechanisms of EV‑mediated communication in inflammatory joint disease.
During local inflammation, infiltrating leukocytes (T cells, B cells, monocytes, monocyte-derived macrophages) and resident synovial macrophages activate fibroblast-like synoviocytes (FLSs) in the synovial membrane by extracellular vesicle (EV)-mediated cell-to-cell communication. Activated FLSs further maintain inflammation by production of cytokines and enzymes. By the release of their own EVs, FLSs signal back to immune cells and enzyme-loaded, FLS-derived EVs can invade aggrecan-rich extracellular matrix (ECM). Leukocyte-derived EVs can carry factors that cause local hypercoagulation. Activated-platelet-derived EVs cause IL-1 receptor-mediated activation of FLSs and can be internalized by activated neutrophils, maintaining the inflammatory phenotype of the two cell types. B cells recog-nize citrullinated proteins in EV-immune complexes, which might be part of the autoimmune process in rheumatoid arthritis. AP1, activator protein 1; CCL2, C–C motif chemokine 2; CCL5, C–C motif chemokine 5; COX-2, cyclooxygenase 2 (prostaglandin G/H synthase 2); DC, dendritic cell; HA, hyaluronic acid; ICAM, intercellular adhesion molecule; JNK, c-Jun N-terminal kinase; MMP, matrix metalloproteinase; PGE, prosta-glandin E; mPGES-1, microsomal prostaglandin E synthase 1; sPLA2-IIA, secreted phospholipase A2-IIA; TLR, Toll-like receptor; VEGF, vascular endothelial growth factor.