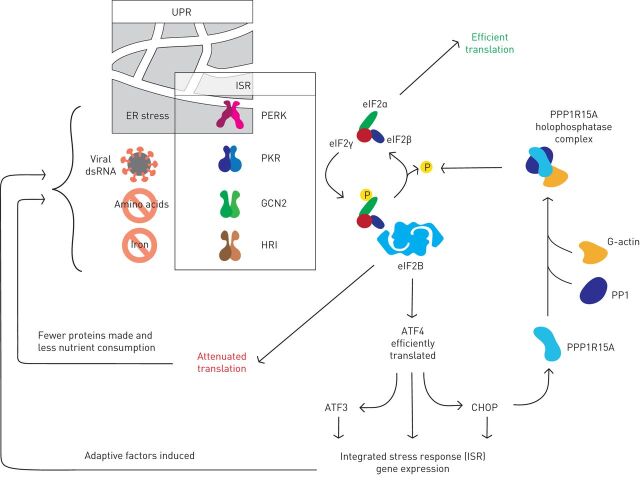
FIGURE 1.
The integrated stress response (ISR) is triggered by stress-sensing kinases that phosphorylate eukaryotic initiation factor (eIF)2α, a component of the eIF2 translation initiation complex. Protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) responds to endoplasmic reticulum (ER) stress, and so the ISR overlaps with the unfolded protein response (UPR). PKR detects viral double-stranded (ds)RNA. General control non-depressible (GCN)2 is activated by amino acid deficiency. Heme-regulated inhibitor (HRI) responds to iron depletion. Phosphorylated eIF2α binds avidly to eIF2β to inhibit most translation, but some mRNAs including those encoding the transcription factors ATF4 and CHOP are translated more efficiently. The resulting gene expression restores homeostasis by enhancing oxidative protein folding in the ER; promoting amino-acyl transfer (t)RNA synthesis; and inducing antioxidant genes. PPP1R15A (also known as GADD34) is eventually induced and in complex with PP1 and G-actin dephosphorylates eIF2α to terminate the ISR.