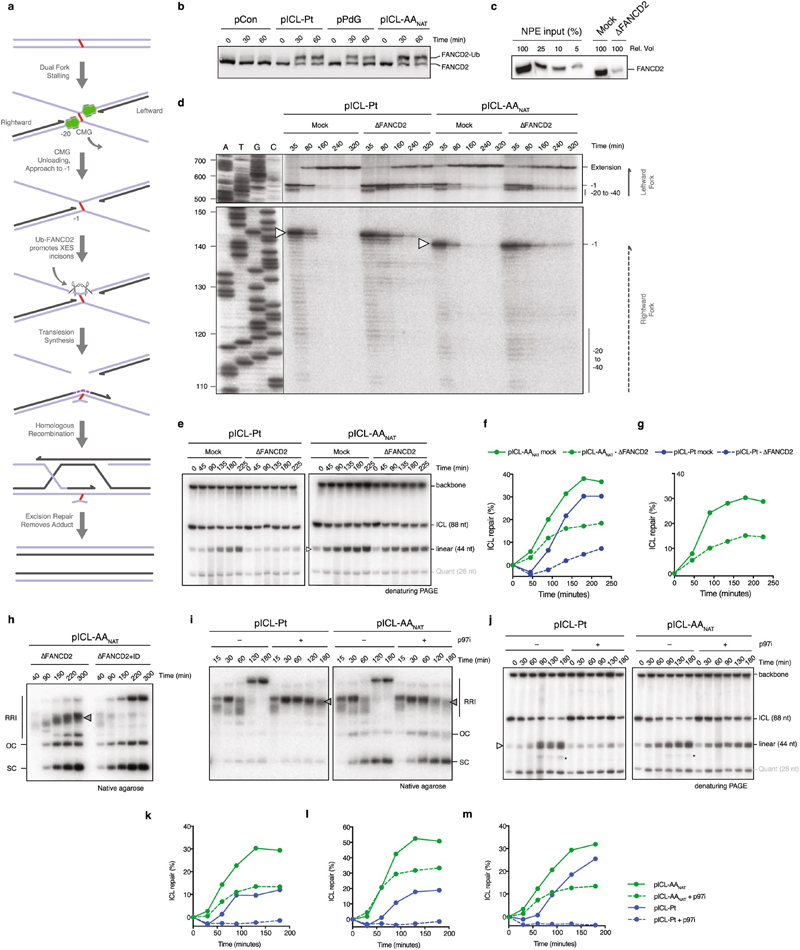
Extended Data Figure 2. Acetaldehyde ICLs are repaired by Fanconi-dependent and independent mechanisms.
(a) Model for ICL repair by the FA pathway. Upon convergence of two replication forks at the crosslink, the CMG helicase is unloaded from the DNA to allow approach of one replication fork to the -1 position. Ubiquitylation of FANCD2 promotes the recruitment of the XPF-ERCC1-SLX4 (XES) complex to the ICL that enables nucleolytic incisions that unhook the crosslink. This step could be preceded by fork reversal of one of the stalled replication forks22. Incisions generate a broken strand and a strand with an adduct, the latter is bypassed by TLS while the broken strand is repaired by homologous recombination. In mammalian cells, it has been shown that a single fork can pass over the ICL without unhooking23, this ‘traverse’ gives rise to a structure that resembles the one generated after fork convergence and CMG unloading and could follow the same steps subsequently. (b) Indicated plasmids were replicated in Xenopus egg extract, reaction samples were analysed by western blot with FANCD2 antibody. Two independent experiments. (c) FANCD2 Western blot showing a titration of Xenopus egg extract compared to mock and FANCD2 depleted extract. Two independent experiments. (d) Indicated plasmids were replicated in Mock or FANCD2 depleted (ΔFANCD2) extract in the presence of α-32P-dCTP. Repair products were digested by AflIII, separated on a sequencing gel alongside a ladder derived from extension primer S, and visualized by autoradiography. White arrow denotes –1 product that is 2 nt larger in pICL-Pt due to the position of the ICL. Three independent experiments. (e) Indicated plasmids were replicated in Mock or FANCD2 depleted (ΔFANCD2) extract, repair intermediates were digested with NotI, 3’ labelled, and resolved by denaturing PAGE. Quantification of repair based on the intensity of 44 nt product is shown in main Fig. 1g. (f) Independent experimental duplicate of Main Fig. 1g. (g) Independent experimental triplicate of Main Fig. 1g, but only using pICL-AANAT. (h) Plasmid pICL-AANAT was replicated in FANCD2 depleted extract, or FANCD2 depleted extract supplemented with recombinant FANCI-FANCD2 complex (ID). Reaction samples were resolved by native agarose gel, visualised by autoradiography. Replication/repair intermediates (RRI), open circle (OC), and supercoiled (SC) products are indicated. Stalled repair product (grey arrow) is indicated. Two independent experiments. (i) Indicated plasmids were replicated in Xenopus egg extract in presence or absence of p97i and the intermediates were resolved by native agarose gel electrophoresis. Stalled repair products (grey arrow) are indicated. Seven independent experiments. (j) The indicated plasmids were replicated in Xenopus egg extracts in presence or absence of p97i and repair intermediates were digested with NotI, 3’ labelled, and resolved by denaturing PAGE. Increase in intensity of the 44 nt band (white arrow) in time indicates ongoing replication and repair. A higher mobility band, probably generated from end-joining activity in some extracts is indicated (*). (k) Quantification of repair based on the intensity of 44 nt product on gel in (j) as indicated in methods (Supplemetary Information Methods). (l) Quantified independent experimental duplicate of (j). (m) Quantified independent experimental triplicate of (j).