Figure 1.
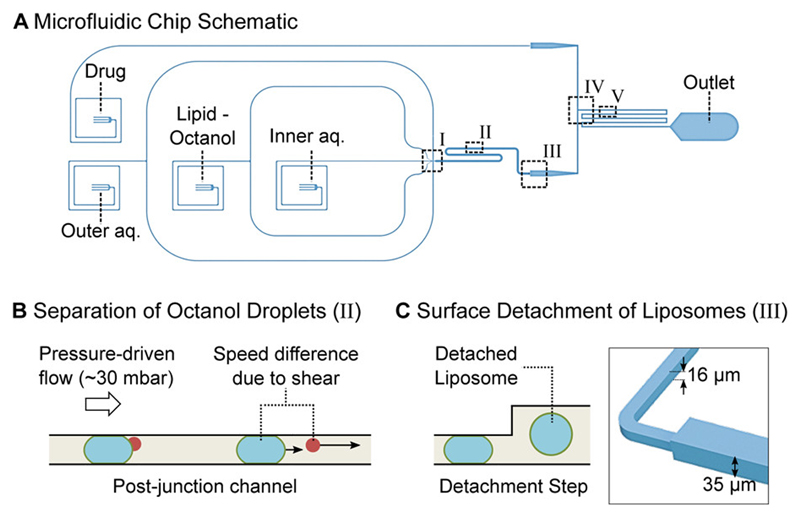
Microfluidic total analysis system for quantifying drug permeability across liposome membranes. A: The microfluidic chip features four inlets, one outlet and two different channel heights. The outer aqueous, inner aqueous and lipid-octanol inlets are needed for liposome production on chip. The fourth inlet is used to flush in the drug whose permeability is to be measured. The liposome production occurs at a 6-way junction, where the aqueous flows meet the lipid-octanol phase (I). The 1-octanol pocket which is initially attached to the liposome separates from it in the post-junction channel within minutes after production (II). After an increase in channel height (III), the liposomes are mixed with the drug solute (IV). The transport measurement takes place as the liposomes flow towards the outlet, immersed in a bath of the autofluorescing drug (V). B: Mechanism to separate the liposome population from the octanol droplets. The liposomes typically have radii of 15-18 μm upon production and octanol droplets of < 8 μm. The channel height post formation is lower than the diameter of the liposomes which leads to a significant difference in velocity for octanol droplets and liposomes as indicated by the arrows. The octanol droplets pass through the device first and are discarded at the outlet. C: Upon production, the liposomes’ diameters are larger than the height of the microfluidic channel. An increase in channel height from 16 μm to 35 μm frees the liposomes from the geometric confinement and enables transport measurements across the membrane without the risk of shear-induced leakage.