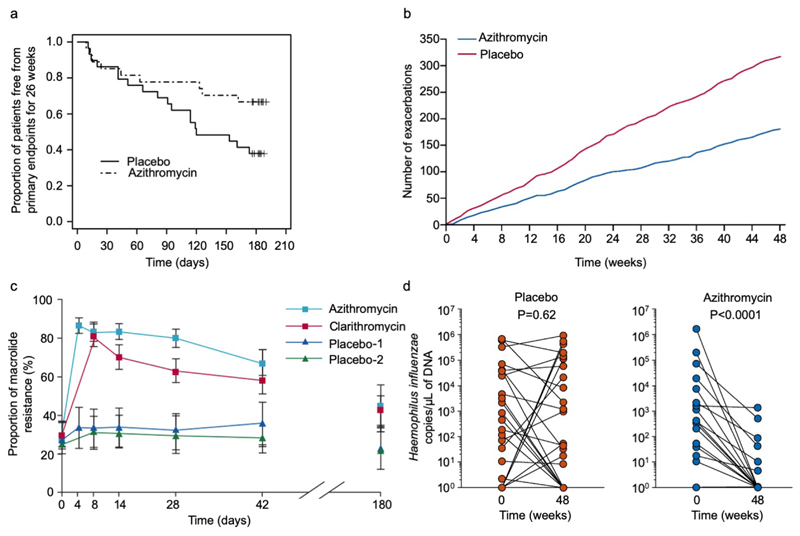
Figure 3. Clinical trial data for azithromycin data in severe asthma and for induction of resistance in healthy volunteers.
(a) Proportion of subjects with non-eosinophilic severe asthma (FeNO<upper limit of normal and a blood eosinophilia ≤200/ml) free from primary endpoints (severe exacerbations) for 26 weeks, according to study group (azithromycin or placebo) in AZISAST[74]. Azithromycin significantly decreased the number of patients with at least one primary endpoint from 9/27 (33%) azithromycin-treated subjects vs 18/29 (62%) placebo-treated subjects; relative risk 0.54, 95% CI 0.29 to 0.98, p=0.037). Reproduced from Brusselle GG and colleagues[74] with permission from BMJ Publishing Group Ltd. (b) Cumulative severe and moderate asthma exacerbations during 48 weeks of treatment with azithromycin 500 mg, three times per week, or placebo in AMAZES[75]. Reproduced with permission from Elsevier. (c) Temporal changes in the proportion of macrolide-resistant streptococci after azithromycin and clarithromycin use in healthy volunteers. Mean data are shown for 204 volunteers (of 224 recruited) assessed to day 42, and for 99 volunteers assessed to day 180. Error bars are 95% CIs. Reproduced from Malhotra-Kumar and colleagues[230] with permission from Elsevier. (d) Haemophilus influenzae copy number before and after either placebo (left, red) or azithromycin (right, blue) in AMAZES. Reproduced from Taylor SL[91] with permission from American Thoracic Society.