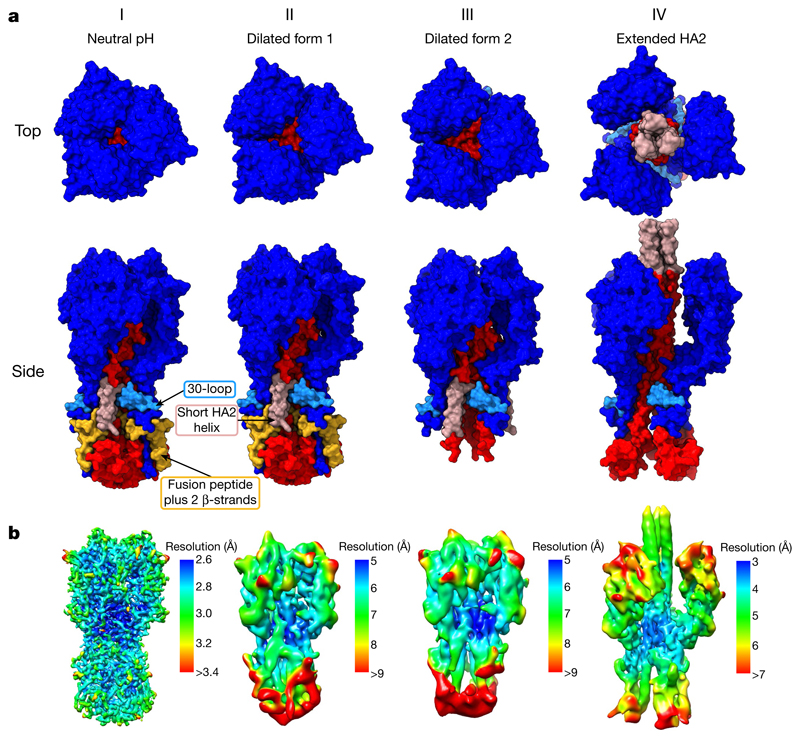
Fig. 2. Surface representations of HA fusion intermediates.
a, Molecular surfaces for states I–IV. HA1 is coloured in dark blue and the 30-loop (residues 22–37) in light blue. HA2 is in red, with residues 1–37 (containing the fusion peptide, residues 1–23) in yellow and the short helix (residues 38–55) in pink. Top views of surfaces show the increasing dilation of the membrane-distal domains. Side views show several features. Between states II and III, the fusion peptide and attached two β-strands are absent while the base of the protein becomes disordered. Between states III and IV, the 150 Å coiled-coil forms between the dilated HA1 domains. The base of HA2 has also opened up in state IV compared with state I. b, Cryo-EM maps, coloured by local-resolution estimations (contour bars are state-specific).